Unit 11 Enthalpy
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
System
chemicals/reacting molecules
Enthalpy (H)
heat energy stored in chemical system
Exothermic
> bond __; energy __; surroundings __heat (more/less)
> __ve ΔH as p__ energy < r__ energy
> e.g. n__, c__, r__
making, released, more, -ve, product, reactant, neutralisation, combustion, respiration
Endothermic
> bond __; energy __; surroundings __heat (more/less)
> __ve ΔH as r__ energy < p__ energy
> e.g. p__, l__, ice packs
breaking, absorbed, less, +ve, reactant, product, photosynthesis, lightning
Activation energy
min amt of energy needed for successful collision
What energy change is this?
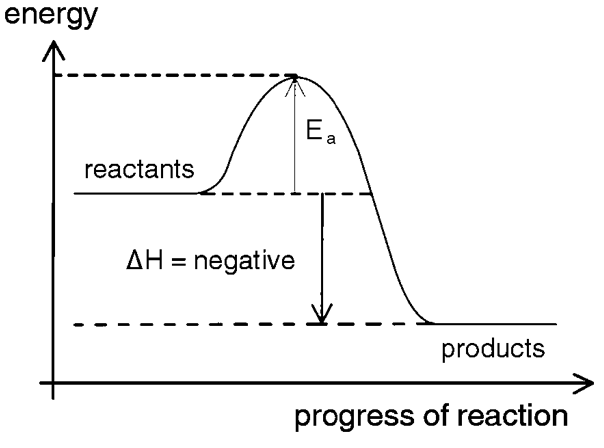
exothermic
What energy change is this?
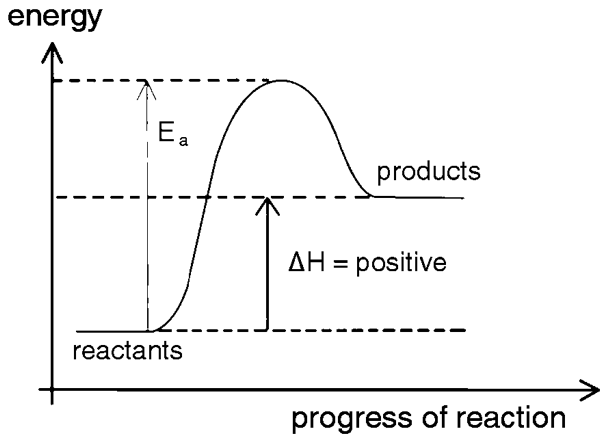
endothermic
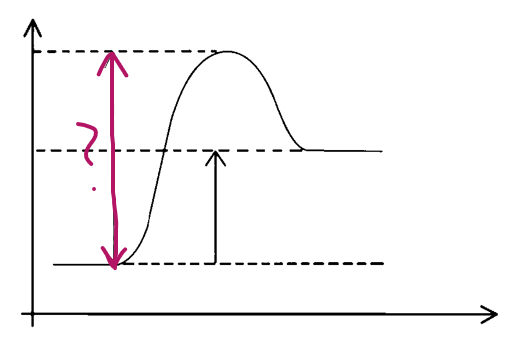
Y axis?
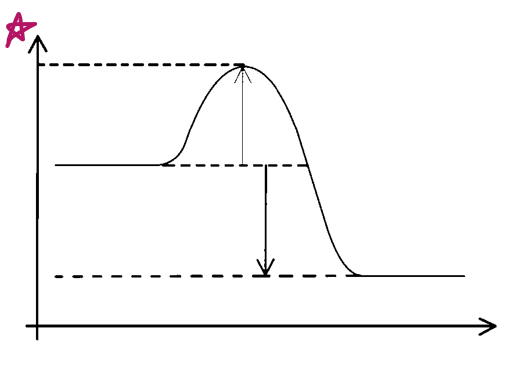
energy
X axis?
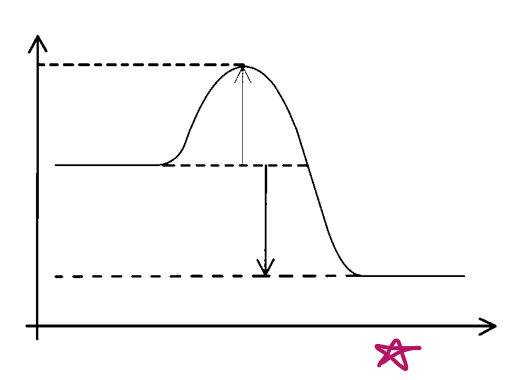
progress of reaction
e__, __
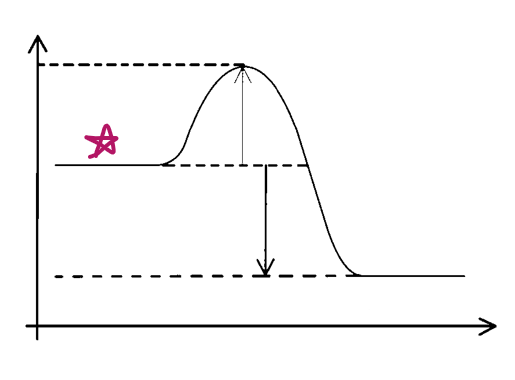
exo, reactants
e__, __ e__
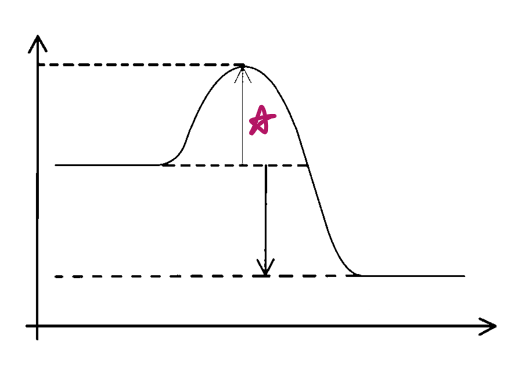
exo, activation energy
e__, __ve Δ__
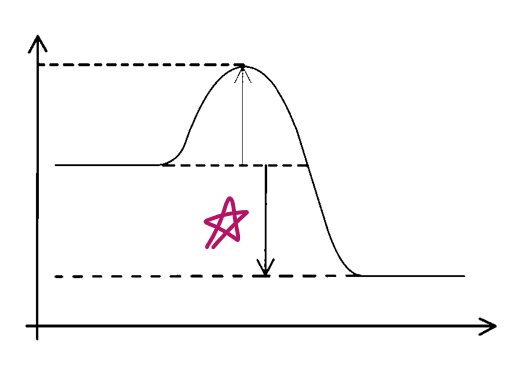
exo, -ve, H
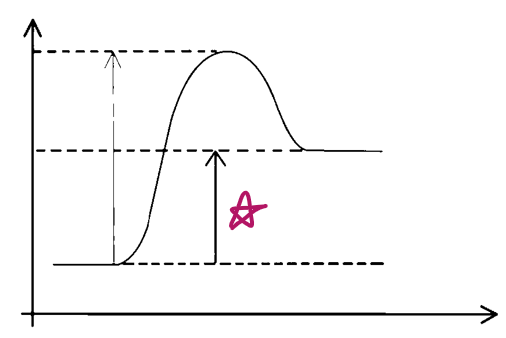
e__, __
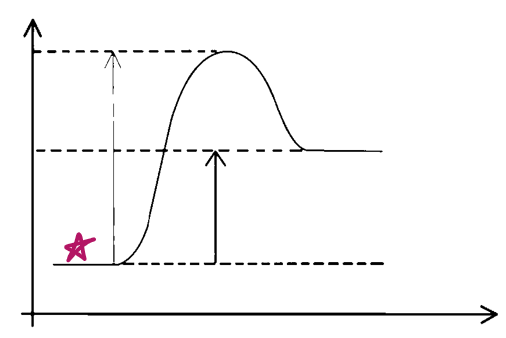
endo, reactants
e__, __ e__
endo, activation energy
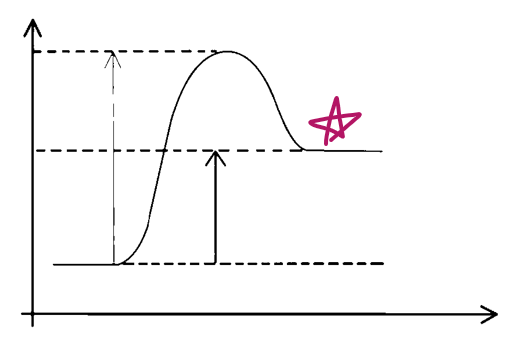
e__, __ve Δ__
endo, +ve, H
e__, __
endo, products
Energy change is measured via a
calorimeter
To find ΔH,
1. use _ = __Δ_
2. ΔH = (_/1000) / _
Q, mcT, Q, n
ΔH unit
kJ/mol
Why Q/1000?
J to kJ
Bond energy
amt of energy needed to break 1 mole of bonds in a molecule to form indiv atoms
↑b__ e__ = s__ c__ bond & ↑energy needed to b__ it & separate atom
bond energy, str covalent, break
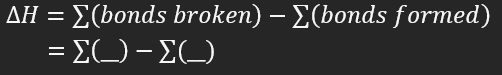
fill in the blank
reactant, product