MD 3. Aldehyde and Ketone Nucleophilic Addition Reactions
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
are aldehydes or ketones more reactive, Why?
aldehydes are more reactive due to sterics and electronics as there is only 1 R group hindering the nucleophilic attack and the carbon will be more electrophilic as it is only affected by one +I group from the singular R group.
Whereas, ketones have two R groups in the way causing more hinderances and two +I groups making the carbonyl C less partially positive
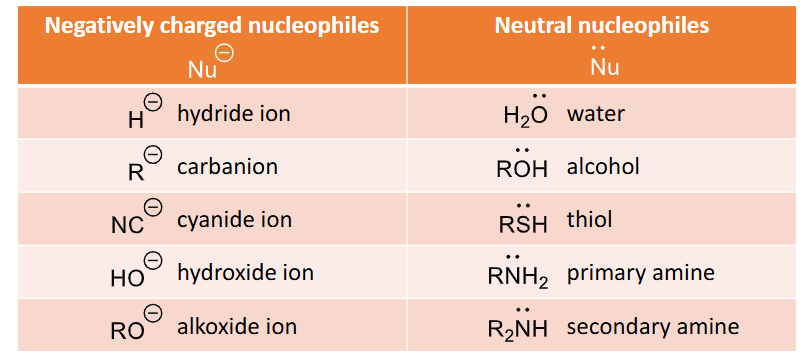
What nucleophiles can be used and the conditions required for each types?
negatively charged nucleophiles - the stronger nucleophiles - E.g. H-, R-, NC-, HO- Ro-
Neutral nucleophiles, an acid catalyst is often used, lone pair is what attacks the carbon, e.g. H2O, ROH, RSH, RNH2, R2NH
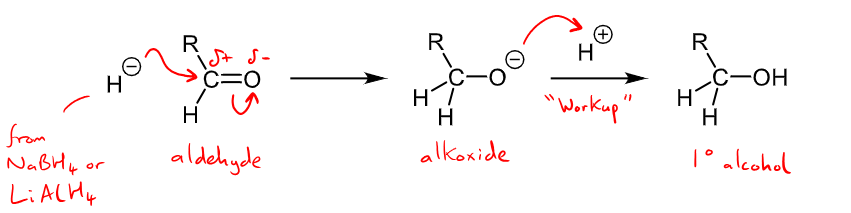
Outline the mechanism and reaction details for the nucleophilic addition of hydrides
results in a reduction to alcohols
Aldehydes will make a primary alcohol and ketones will produce a secondary alcohol.
H- in step one comes from a hydride reducing agent - LiAlH4 is a stronger reducing agent but NaBH4 also used
H+ in work up/step 3 comes from aqueous acid
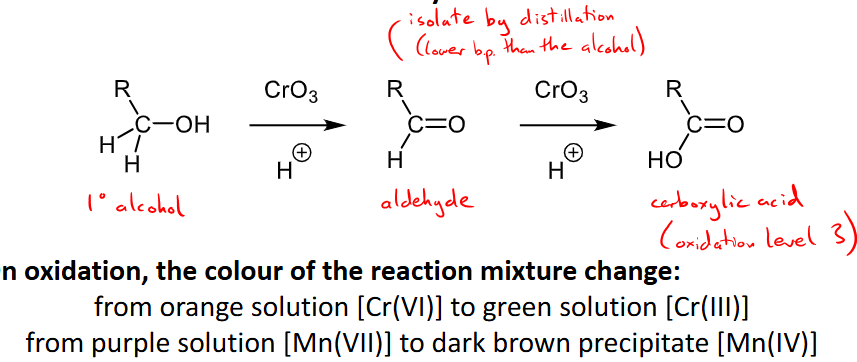
Outline the mechanism and reaction details for the oxidation of alcohols to carbonyls.
oxidising agents can be chromium (VI) oxide, CrO3, potassium manganate(VII) KMnO4, acidified potassium dichromate Na2Cr2O7
secondary alcohols will become ketones, primary will become aldehydes (and then oxidised again to a carboxylic acid), tertiary are very hard to oxidise as a C-C bond must be broken.
oxidation colour change of orange to green for chromate solutions or purple to dark brown for manganate solutions.
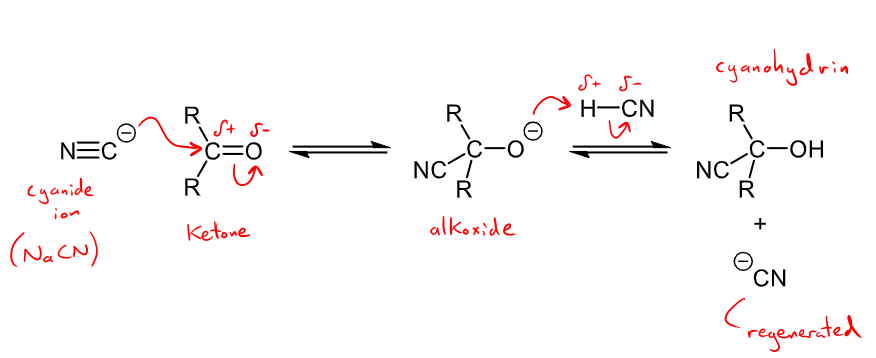
Outline the mechanism and reaction details for the nucleophilic addition of a cyanide ion
Reaction with NaCN and HCN forms a cyanohydrin
Cyanohydrins are useful as they can be converted to other functional groups
Cyanide ion from NaCN attacks carbonyl, alkoxide is formed and the H from H-CN reacts with the O- to form the alcohol group and the NC- group is regenerated.
What are cyanohydrins commonly used for?
good as they can be converted to other functional groups
can be hydrolysed into alpha-hydroxy carboxylic acids for the cosmetic industry
or plants and insects can react then with enzymes will form a ketones and HCN (poison) as a defence mechanism
also key intermediates with aromatics for the synthesis of pesticides
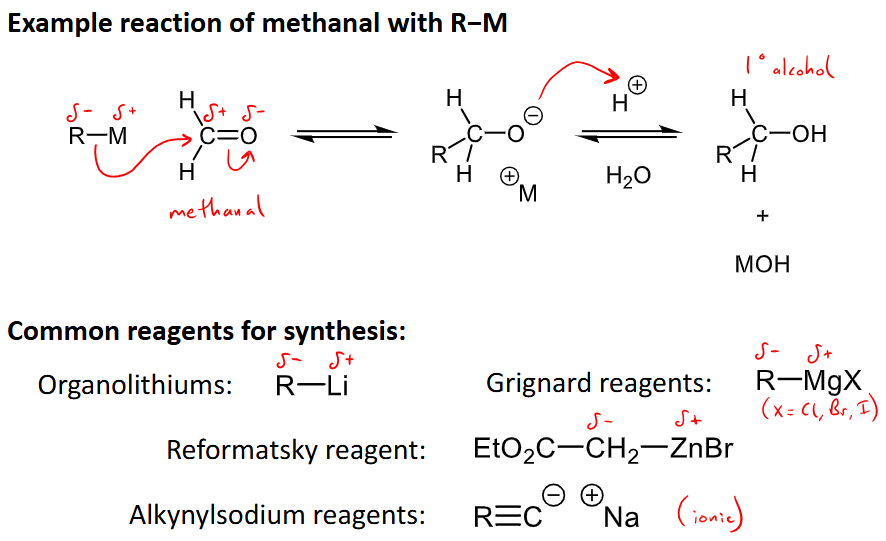
What is the general reaction for the nucleophilic addition with organometallics?
Common reagents: R-Li (organolithiums), R-MgX (Grignard reagents), EtO2C-CH2-ZnBr (Reformatsky reagent), R=-C-+Na (alkynylsodium reagents, ionically bonded)
How is the Grignard Reagent prepared?
R-X + Mg to form R-MgX
Dry diethyl ether solvent under and inert atmosphere of N2 or Ar gas (as they react with oxygen)
also must be dry/anhydrous as Grignard reagents deprotonate water to make an alkane.
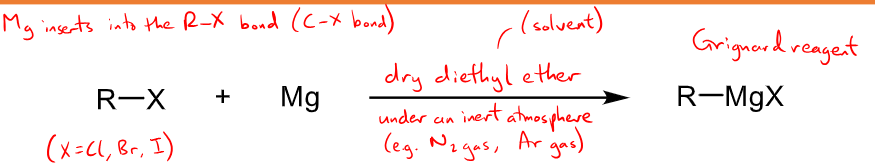
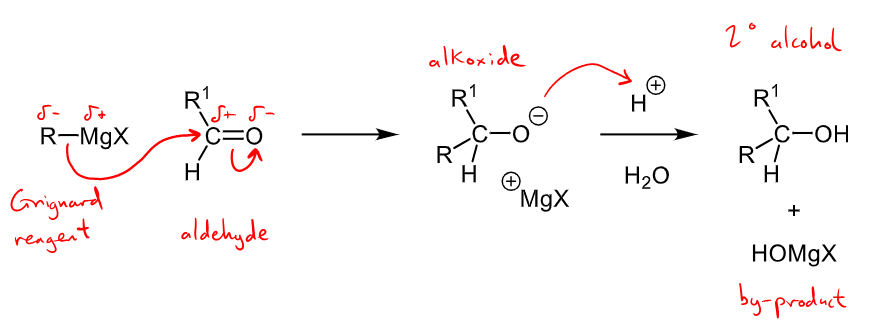
Outline the mechanism of a ketone/aldehyde with Grignard Reagent and one of its synthesis uses in the med/pharm context?
R-MgX attacks C and forms an alkoxide, alkoxide reacts with a proton to form an alcohol with HOMgX by-product
secondary alcohol formed if aldehyde reacts, tertiary alcohol formed if ketone reacts.
used in the synthesis of tamoxifen, an oestrogen receptor modulator used in breast cancer treatment.
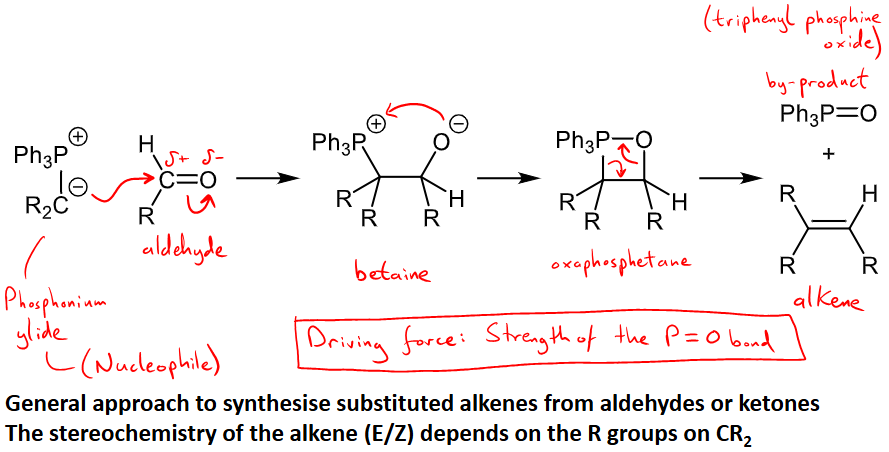
Outline the mechanism for a reaction with phosphonium ylides (Wittig reaction)
phosphonium ylides = R2C=PPh3
Form alkenes int he Wittig reaction
alkenes and Ph3P=O (triphenyl phosphine oxide) by-product formed
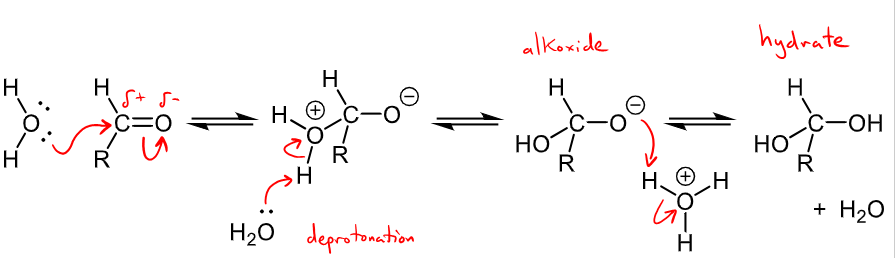
Outline the mechanism for the reaction with water (hydration)
H2O is an oxygen nucleophile, water adds reversible to aldehydes and ketones - EVERY STEP IS REVERSIBLE
water is a weak nucleophile and adds slowly to the C=O
hydrates are known as 1,1, diols or geminal diols
Addition rate can be increased by addition of HO- base or H+ acid catalyst
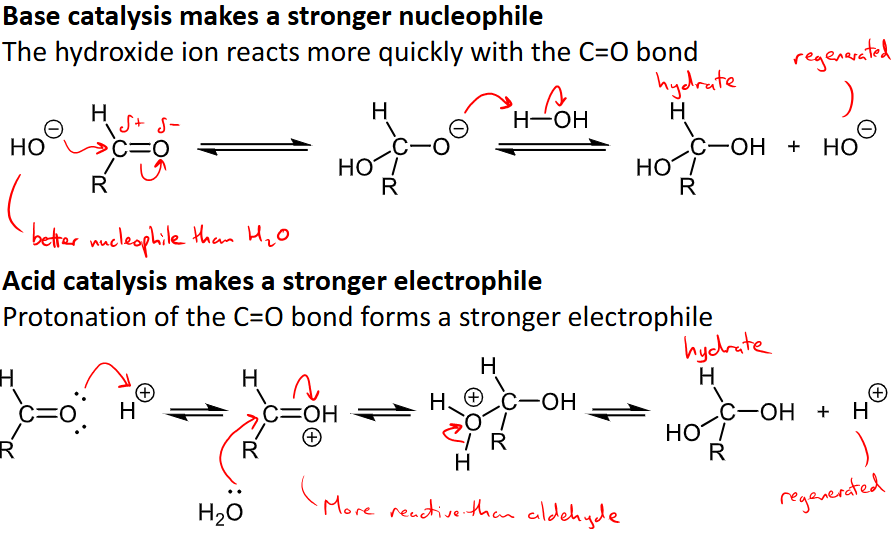
How does acid/base catalysis affect the addition reaction?
base catalysis makes a stronger nucleophile and hydroxide ion reacts quicker with C=O
Acid catalysis makes a stronger electrophile through the protonation of C=O
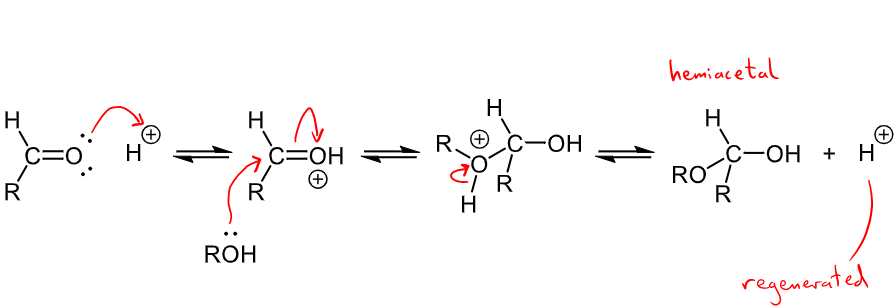
Outline the mechanism for the Addition of an alcohol
alcohols are nucleophiles that add reversibly to form hemiacetals - every step is reversible
Acid catalyst used to increase rate of addition
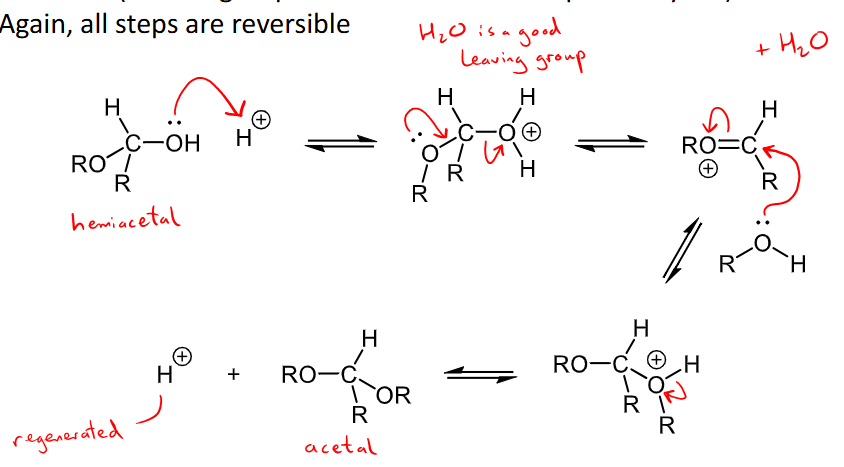
Outline the Mechanism for the formation of an Acetal
hemiacetals can react with another equivalent of an alcohol to form an acetal - the OH group of the hemiacetal is replaced by another OR in an acetal
every step is reversible
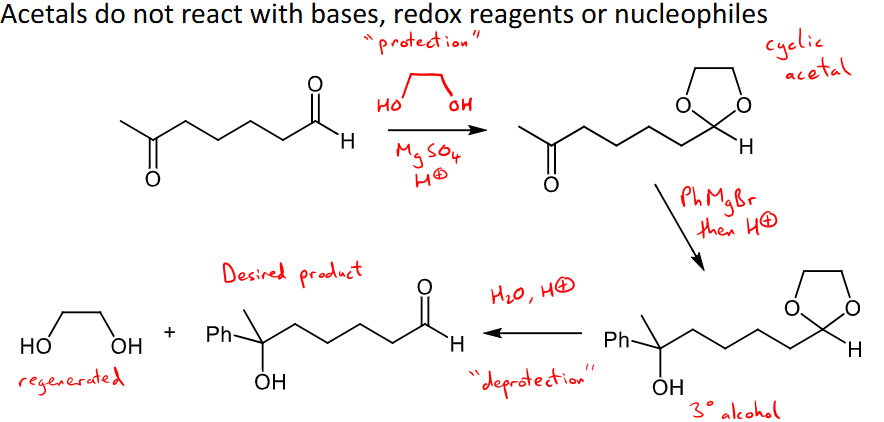
How can acetals be used as protecting groups?
acetals do not react with bases, redox reagents or nucleophiles and there can be added to form a cyclic acetal over a carbonyl to ensure only a portion of your molecule is actually reacting
then adding water and H+ can cause a ‘deprotection’ step that regenerates the acetal.
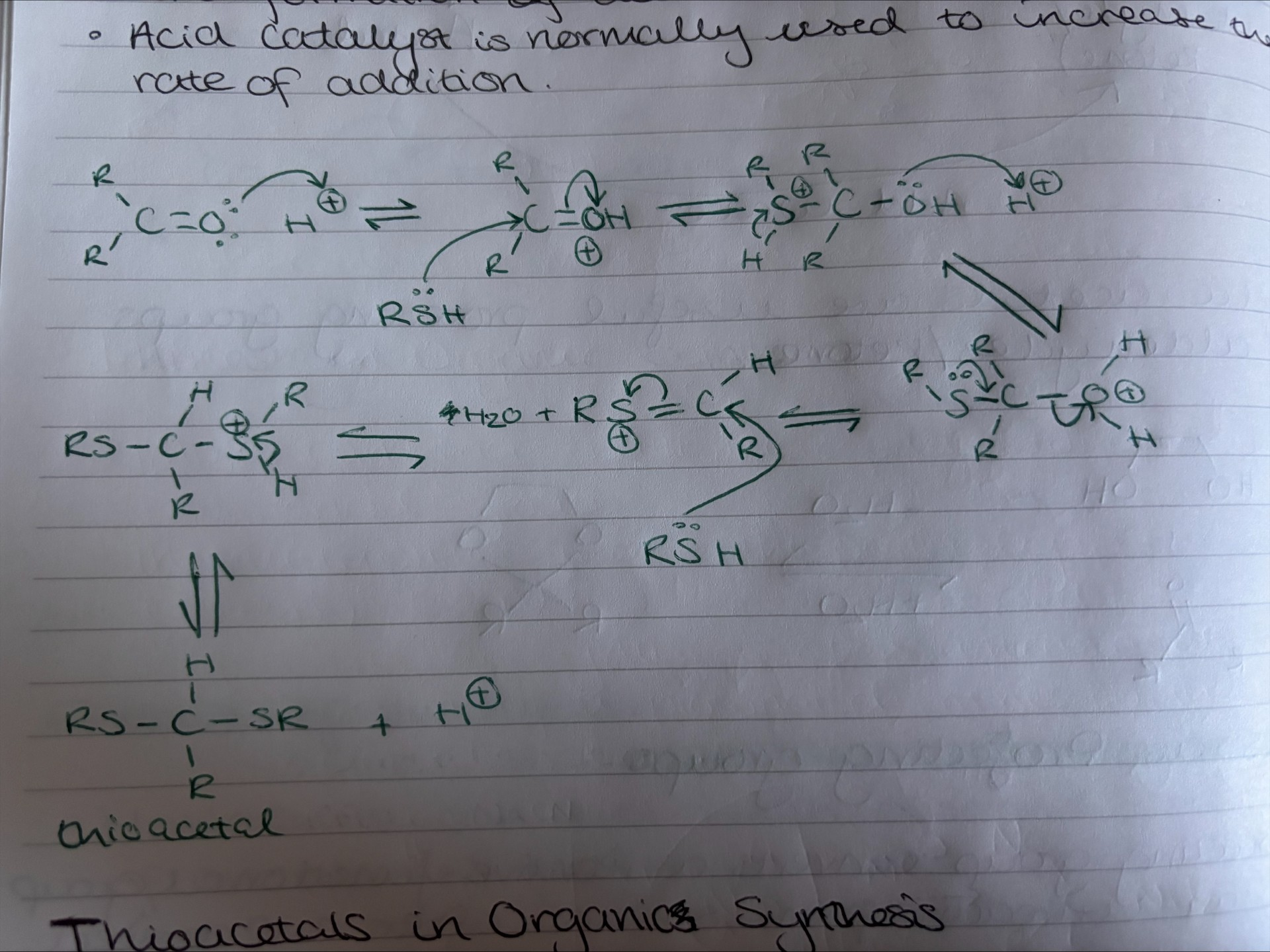
Outline the mechanism for the addition of a thiol
thiol adds reversibly to ketones/aldehydes (EVERY STEP REVERSIBLE)
acid catalyst usually used to increases rate of addition.
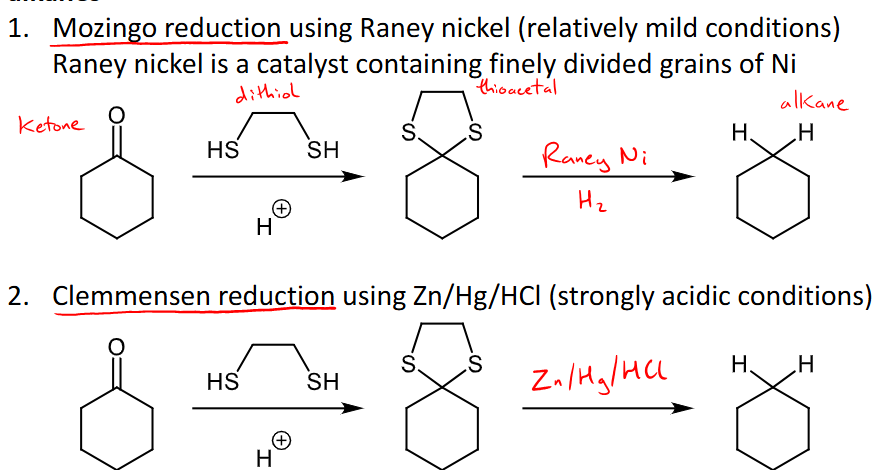
Why are thioacetals useful in organic synthesis?
They can reduced to alkanes, add the dithiol and it forms thioacetal where the carbonyl once was and then the specific conditions are added to reduce it to an alkene.
Mozingo reduction uses raney nickel as a catalyst to form an alkane from the thioacetal
Clemmensen reudction uses Zn/Hg/HCl (very strongly acidic conditions) to reduce to alkanes from thioacetal
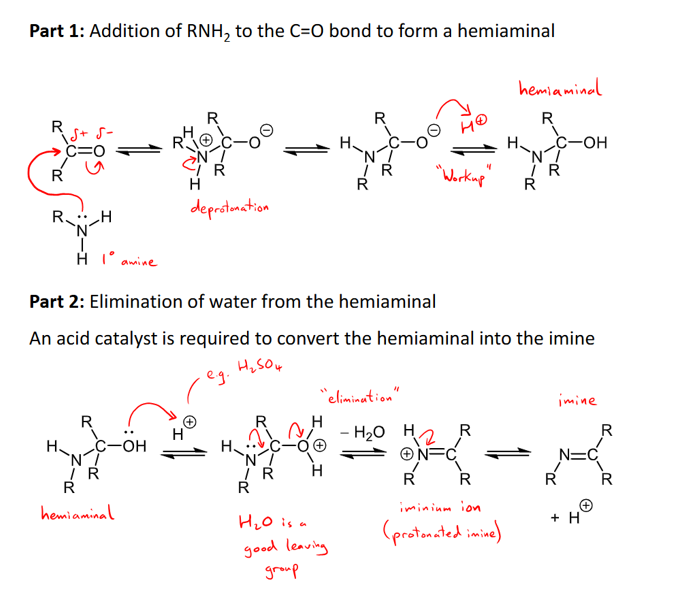
Outline the mechanism and conditions for the addition of a primary amine/formation of an imine
primary amine adds reversibly to form an imine
water is a by-product so it is also a condensation reaction.
Happens in two parts: 1) addition of RNH2 to form hemiaminal, 2) elimination of water from hemiaminal to form imine
amines are strong nucleophiles and does NOT need an acid catalyst for part 1.
Part 2 needs an acid catalyst to convert hemiaminal to imine.
Optimum pH for reaction is around 4.5
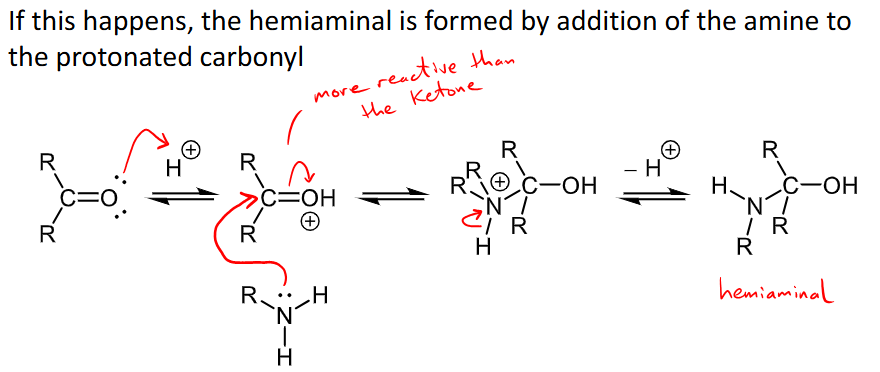
How can the pH affect the formation of an imine?
an acid catalyst is not needed for step 1, but the C=O may be protonated at pH 4.5 and the hemiaminal is formed through addition of the amine to the protonated carbonyl - creating a new potential mechanism for part 1 - this protonated carbonyl is actually more reactive than the ketone
if the pH is under 4.5 the amine will be protonated and not act as a nucleophile
if the pH is above 4.5 there is not enough H+ to protonate the OH in the hemiaminal
this is why 4.5 is optimum.
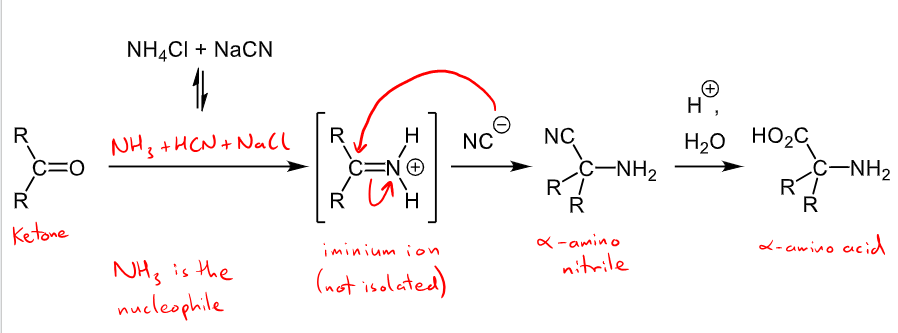
How are imines used in organic synthesis?
they can react with nucleophiles to form other functional groups - the aprtially positive carbon in the C=N is attacked
E.g. Strecker synthesis of alpha amino acids
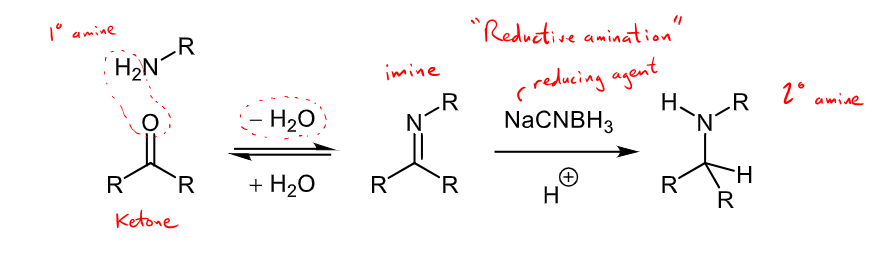
What is reductive amination?
imines being reduced to form amines
reductive amination forms an amine from and aldehyde/ketone through an intermediate imine
NaBH4 or NaCNBH3 can be used as reducing agents
the secondary amine produced can then react with another aldehyde/ketone to form a tertiary amine through another reductive amination.
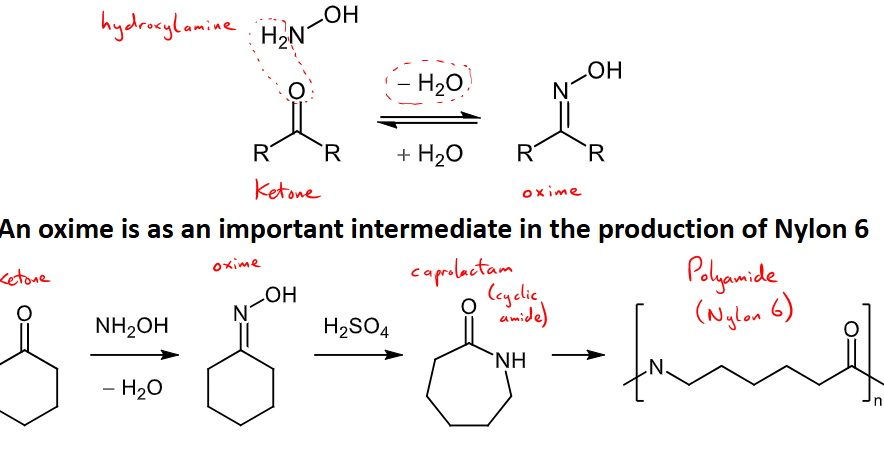
How are oximes formed?
formed through the reaction of an aldehyde/ketone with hydroxylamine
oximes are important intermediates in the production of nylon 6
mechanism follows that of imine formation.
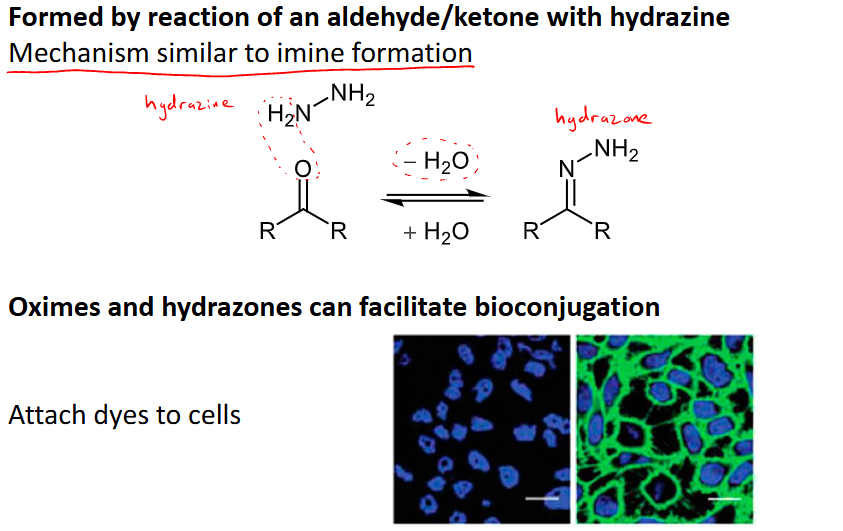
How is a hydrazone formed?
reacting an ald/ket with hydrazine
mechanism follows that of imine formation