Gibbs Free Energy and Entropy: Thermodynamics of Melting and Solubility
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
What is the equation for Gibbs Free Energy?
DGrxn = DHrxn - T DSrxn
When is a reaction thermodynamically favored?
When DHrxn is negative and DSrxn is positive.
What happens when DHrxn is positive and DSrxn is negative?
The reaction is never favored.
What does it mean if DHrxn is positive and DSrxn is positive?
The reaction is favored at high temperatures.
What does it mean if DHrxn is negative and DSrxn is negative?
The reaction is favored at low temperatures.
What is the enthalpy change for the reaction between N2 and H2 to produce NH3?
DHrxn = -91.8 kJ/mol.
What is the entropy change for the reaction between N2 and H2 to produce NH3?
DSrxn = -199 J mol-1 K-1.
What is the Gibbs Free Energy change for the reaction between N2 and H2 to produce NH3 at 25.0 °C?
DG°rxn = -32500 J mol-1.
Is ice melting an exothermic or endothermic process?
Endothermic.
Why does ice melt above 0 °C despite being endothermic?
Because entropy dominates enthalpy, making the process thermodynamically favored.
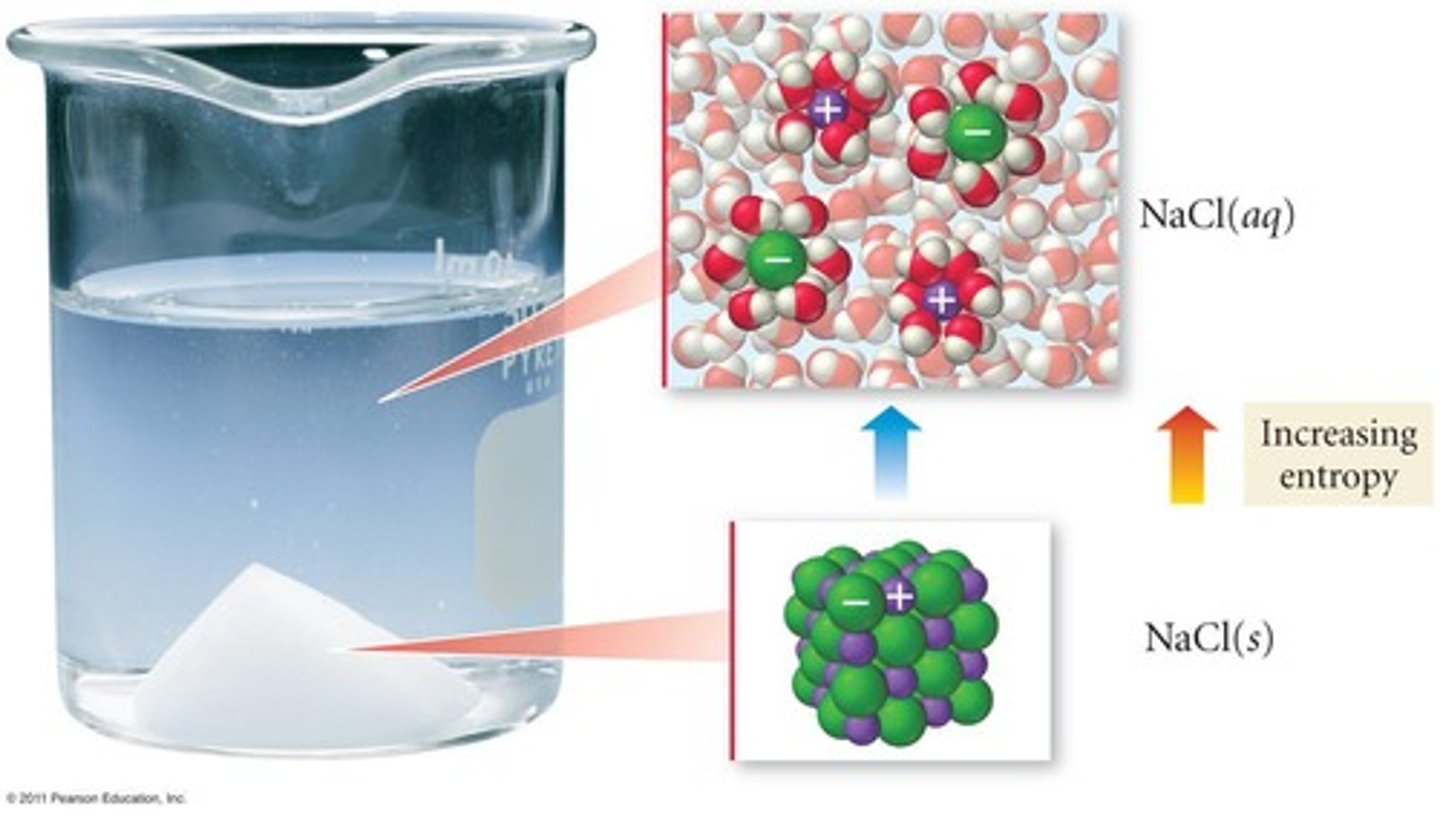
What happens to the solubility of salts like NaCl when heated?
Solubility increases with temperature.
What happens to the solubility of gases when heated?
Solubility decreases with temperature.
What is the entropy change for a reaction favoring reactants with 3 mol gas to 1 mol gas?
DSrxn = Negative.
What is the entropy change for a reaction favoring products with 1 mol gas to 2 mol gas?
DSrxn = Positive.
What is the relationship between temperature and entropy favoring reactants?
As temperature increases, the reaction is less favored.
What is the relationship between temperature and entropy favoring products?
As temperature increases, the reaction is more favored.
What is the significance of entropy (DS) in thermodynamics?
Energy and molecules want to disperse.
What is the order of entropy from solid to gas?
Ssolid < Sliquid < Ssolution << Sgas.
How does temperature affect DSrxn?
As temperature increases, DSrxn becomes amplified.
What is the effect of breaking hydrogen bonds on ice melting?
It is enthalpically unfavored.
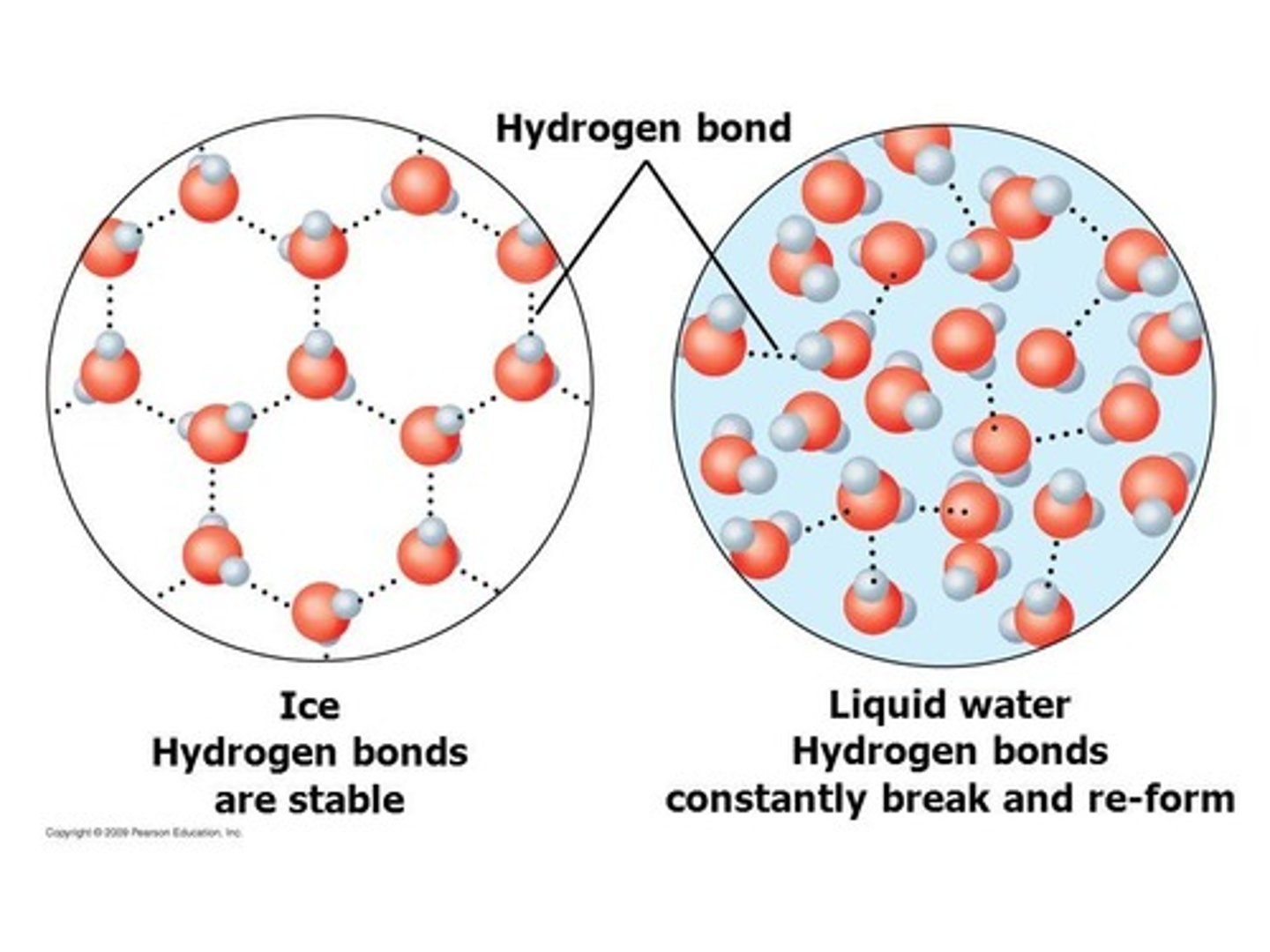
What is the impact of entropy on the melting of ice?
Ice melting is entropically favored.
What is the thermodynamic favorability of ice melting below 0 °C?
Ice melting is unfavored and stays solid.
What is the thermodynamic favorability of ice melting above 0 °C?
Ice melting is favored due to entropy.