Chapter 2: Immunity and Disease
0.0(0)
Card Sorting
1/86
Earn XP
Description and Tags
Last updated 1:14 PM on 10/17/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
87 Terms
1
New cards
Immunity
the body’s ability to resist infectious disease
2
New cards
How does the immune system protect the body?
It distinguishes “self” and “nonself”. The immune system coexists peacefully with the body’s own cells because it displays “self” molecules. However, the immune system responds to cells and substances that are not from the body (“nonself”).
3
New cards
Antigen
any foreign substances that, when introduced into the body, is recognized as “nonself” and activates the immune system.
4
New cards
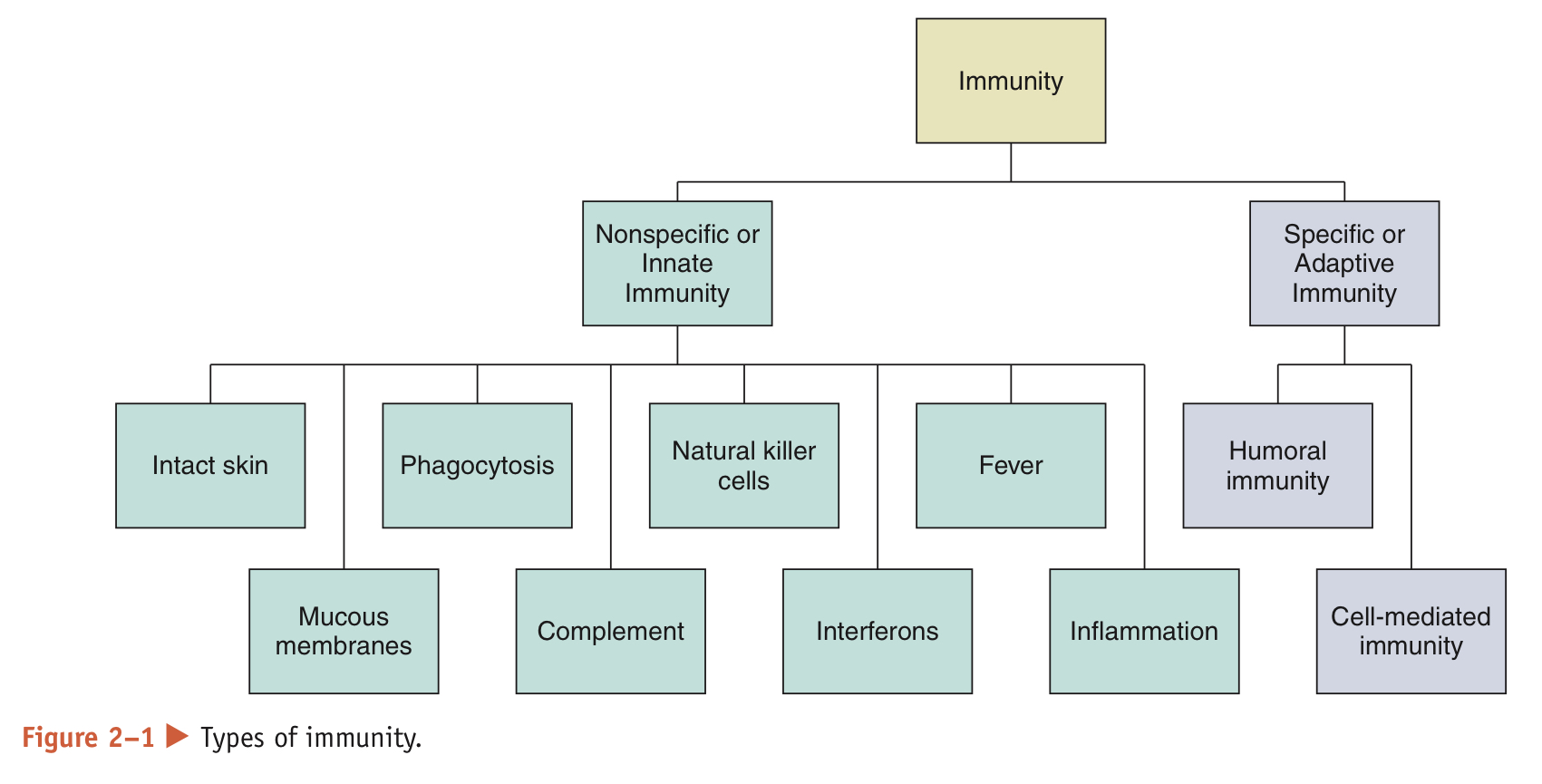
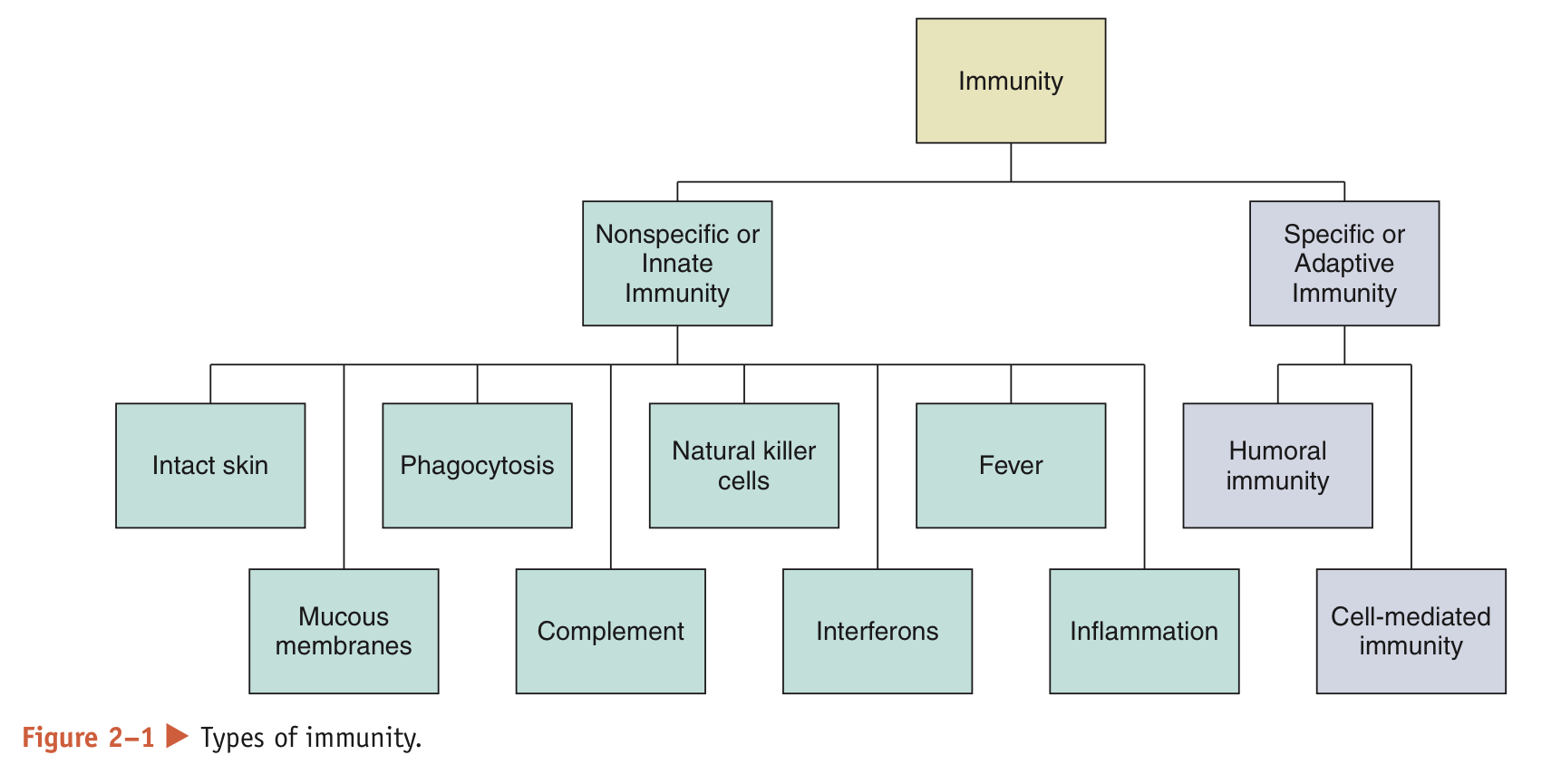
What does resistance to infection rely on?
It relies on 2 types of immunity: nonspecific and specific immunity. Together, they comprise the immune system organs and white blood cells dispersed around the body that are responsible for providing immunity
5
New cards
What is the difference between nonspecific and specific immunity?
Nonspecific: always prepared to defend the body against disease
Specific: must be primed by an initial exposure to an antigen before it can protect the body against disease caused by that particular antigen
Specific: must be primed by an initial exposure to an antigen before it can protect the body against disease caused by that particular antigen
6
New cards
Nonspecific Immunity (innate immunity)
* present at birth and provides immediate, short-term protection against any antigen
* prevents entry and spread of disease causing microorganisms by physical barriers such as intact skin and mucous membranes as well as cellular and chemical defenses
* prevents entry and spread of disease causing microorganisms by physical barriers such as intact skin and mucous membranes as well as cellular and chemical defenses
7
New cards
Pathogen
disease-causing microorganisms
8
New cards
Physical barriers that protect the body from pathogens:
* Skin: prevents entry of the pathogens and other harmful substances into the body. Also produces chemical barriers. secretions like tears, saliva, swear, and sebum contain chemicals that destroy foreign invaders
* Mucous membrane: lines all the body passages open to the exterior and produce mucus, which traps foreign material and forms a barrier to invasion. Microscopic cilia hairs that line the respiratory tract sweep out debris and pathogens trapped in mucus
* Mucous membrane: lines all the body passages open to the exterior and produce mucus, which traps foreign material and forms a barrier to invasion. Microscopic cilia hairs that line the respiratory tract sweep out debris and pathogens trapped in mucus
9
New cards
Phagocytosis
Leukocytes (white blood cells) like macrophages and neutrophils engulf and destroy pathogens in this process
10
New cards
Phagocytes
leukocytes like:
macrophages; below the epidermis and mucous membranes in many tissues
neutrophils; in blood but leave the blood and enters tissues at sites of injury and infection
macrophages; below the epidermis and mucous membranes in many tissues
neutrophils; in blood but leave the blood and enters tissues at sites of injury and infection
11
New cards
Complement
* a group of plasma proteins that assist in the destruction of foreign cells
* circulate in an inactive state, but when complement becomes attached to bacteria cells it becomes active
* Activation results in a cascade of biochemical reactions leading to lysis
* Activation attracts phagocytes to the area and enhances the inflammatory responses
* part of nonspecific immunity, but helps both immunities
* circulate in an inactive state, but when complement becomes attached to bacteria cells it becomes active
* Activation results in a cascade of biochemical reactions leading to lysis
* Activation attracts phagocytes to the area and enhances the inflammatory responses
* part of nonspecific immunity, but helps both immunities
12
New cards
lysis
rupturing the cell membrane
13
New cards
natural killer cells
* type of leukocyte that recognize and eliminate virus-infected cells and cancer cells
* not phagocytic
* secrete chemicals that cause pores to form in the membrane of a target cell, leading to its death
* not phagocytic
* secrete chemicals that cause pores to form in the membrane of a target cell, leading to its death
14
New cards
Interferons
* antiviral proteins produced by some animals cells after viral infection
* stimulate nearby uninfected cells to resist viral infection
* increase the activity of macrophages and antural killer cells
* used to treat infections (hepatitis B and C) and some cancers (melanoma and Kaposi’s sarcoma)
* stimulate nearby uninfected cells to resist viral infection
* increase the activity of macrophages and antural killer cells
* used to treat infections (hepatitis B and C) and some cancers (melanoma and Kaposi’s sarcoma)
15
New cards
Fever
* an abnormally high body temperature which is a systemic response to an infection
* Benefits: slowing growth rate of some pathogens, increasing the effect of interferons, enhancing phagocytosis, stimulating antibody production, and accelerating tissue repair
* Should not always be eliminated; however should be monitored closely
* Very high fevers can cause dehydration, nausea, disorientation, hallucination, seizures, and convulsions
* Benefits: slowing growth rate of some pathogens, increasing the effect of interferons, enhancing phagocytosis, stimulating antibody production, and accelerating tissue repair
* Should not always be eliminated; however should be monitored closely
* Very high fevers can cause dehydration, nausea, disorientation, hallucination, seizures, and convulsions
16
New cards
Inflammation (inflammatory response)
* can be triggered by infection, trauma, intense heat, and chemicals
* Prevents the spread of pathogens, disposes of cell debris and pathogens, and aids in repair of damaged tissue
* damaged cells release potent chemical signals including histamines and kinins.
* these chemicals cause blood vessels in the area to dilate and become more permeable, which causes the cardinal signs and symptoms of inflammation
* Cardinal signs and symptoms: redness, heat, swelling, and prain
* phagocytes are also attracted to the area and clotting proteins are activated and begin to wall off the damaged area
* debris and dead and dying cells may accumulate, forming pus
* Prevents the spread of pathogens, disposes of cell debris and pathogens, and aids in repair of damaged tissue
* damaged cells release potent chemical signals including histamines and kinins.
* these chemicals cause blood vessels in the area to dilate and become more permeable, which causes the cardinal signs and symptoms of inflammation
* Cardinal signs and symptoms: redness, heat, swelling, and prain
* phagocytes are also attracted to the area and clotting proteins are activated and begin to wall off the damaged area
* debris and dead and dying cells may accumulate, forming pus
17
New cards
Specific immunity
(adaptive immunity)
(adaptive immunity)
* responds to antigens of specific pathogens
* once it encounters and responds to an antigen, the body is able to respond quickly to future exposures to the same antigen
* this permits the body to protect itself from infection during subsequent exposures to that pathogen
* once it encounters and responds to an antigen, the body is able to respond quickly to future exposures to the same antigen
* this permits the body to protect itself from infection during subsequent exposures to that pathogen
18
New cards
immunologic memory
ability to remember past encounters with pathogens
(specific immunity)
(specific immunity)
19
New cards
What does adaptive immunity include?
humoral immunity and cell-mediated immunity
20
New cards
humoral immunity
* due to the action of antibodies, which are proteins produced by white blood cells called B lymphocytes (or B cells).
* antibodies provide a defense against extracellular antigens such as bacterial toxins and bacterial cells
* antibodies provide a defense against extracellular antigens such as bacterial toxins and bacterial cells
21
New cards
immunoglobulins
antibodies
22
New cards
cell-mediated immunity
* provides a defense against viruses, abnormal cells, and other intracellular pathogens, and it is the arm of the immune system responsible for rejecting tissue grafts and organ transplants
* T lymphocytes (or T cells) are responsible for cell mediated immunity
* T lymphocytes (or T cells) are responsible for cell mediated immunity
23
New cards
Where do T cells and B cells originate?
The both originate in the red bone marrow
* Immature T cells leave the bone marrow and enter the thymus where they develop the ability to react w/ a unique antigen
* Immature B cells develop their ability to recognize unique antigens in the red bone marrow
Once T and B cells know how to recognize antigens, they leave the thymus and spleen where they wait to be activated by their antigen
* Immature T cells leave the bone marrow and enter the thymus where they develop the ability to react w/ a unique antigen
* Immature B cells develop their ability to recognize unique antigens in the red bone marrow
Once T and B cells know how to recognize antigens, they leave the thymus and spleen where they wait to be activated by their antigen
24
New cards
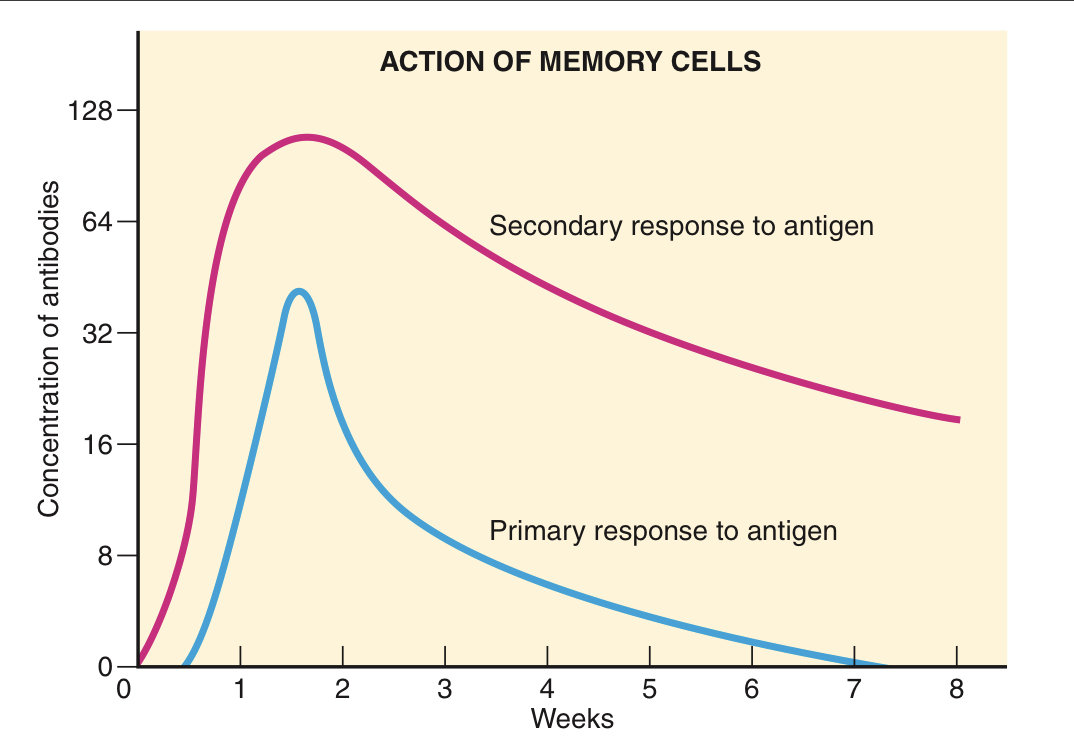
What are memory B cells responsible for?
Memory B cells are responsible for a more potent and rapid antibody response during subsequent exposures to the same antigen. This secondary response to the antigen produces antibodies faster and in larger quantities, and lasts longer than the initial response
25
New cards
What triggers humoral immunity?
* The first exposure to an antigen triggers humoral immunity.
1. Extracellular antigens directly activate a B cell by binding to a receptor on the surface of the B cell.
2. The activated B cell divides and develops into plasma cells and long-lived memory B cells.
3. Plasma cells live about 4 or 5 days and secrete antibodies. Antibodies (immunoglobulins) bind to antigens, making them easier targets for phagocytes and complement. There are several types of immunoglobulins (Ig), each with specialized functions.
1. Extracellular antigens directly activate a B cell by binding to a receptor on the surface of the B cell.
2. The activated B cell divides and develops into plasma cells and long-lived memory B cells.
3. Plasma cells live about 4 or 5 days and secrete antibodies. Antibodies (immunoglobulins) bind to antigens, making them easier targets for phagocytes and complement. There are several types of immunoglobulins (Ig), each with specialized functions.
26
New cards
What are the different immunoglobulins and their functions?
* IgG: Principal component of the primary and secondary response to an antigen. Crosses the placenta and protects the fetus. Activates complement.
* IgM: First antibody produced in the primary response to the antigen. Activates complement.
* IgA: Protects mucosal surfaces by interfering with the ability of pathogens to adhere to cells.
* IgE: Stimulates release of histamine and other chemicals that mediate inflammation and allergic responses.
* IgD: Activates B cells.
* IgM: First antibody produced in the primary response to the antigen. Activates complement.
* IgA: Protects mucosal surfaces by interfering with the ability of pathogens to adhere to cells.
* IgE: Stimulates release of histamine and other chemicals that mediate inflammation and allergic responses.
* IgD: Activates B cells.
27
New cards
What activates cell-mediated immunity?
Like humoral immunity, the first exposure to an antigen triggers cell-mediated immunity
1. A **helper T cell** (also called a CD4 cell) becomes activated by an antigen that was engulfed and digested by a phagocyte and presented to the helper T cell.
2. The activated helper T cell divides, producing additional identical helper T cells (clones) and long- lived memory T cells.
3. The helper T cell clones stimulate antibody production by plasma cells, increase phagocytosis, and stimulate cytotoxic T cells and natural killer cells.
* Memory T cells can rapidly mobilize should the same antigen be encountered again.
* **Cytotoxic T cells** (also called CD8 cells) are activated by antigens displayed on infected cells, abnormal cells, and transplanted organs and tissues.
* In response to these antigens, an activated cytotoxic T cell divides and produces clones and memory T cells. Cytotoxic T cells kill infected and abnormal cells and also kill transplanted organs and tissues
1. A **helper T cell** (also called a CD4 cell) becomes activated by an antigen that was engulfed and digested by a phagocyte and presented to the helper T cell.
2. The activated helper T cell divides, producing additional identical helper T cells (clones) and long- lived memory T cells.
3. The helper T cell clones stimulate antibody production by plasma cells, increase phagocytosis, and stimulate cytotoxic T cells and natural killer cells.
* Memory T cells can rapidly mobilize should the same antigen be encountered again.
* **Cytotoxic T cells** (also called CD8 cells) are activated by antigens displayed on infected cells, abnormal cells, and transplanted organs and tissues.
* In response to these antigens, an activated cytotoxic T cell divides and produces clones and memory T cells. Cytotoxic T cells kill infected and abnormal cells and also kill transplanted organs and tissues
28
New cards
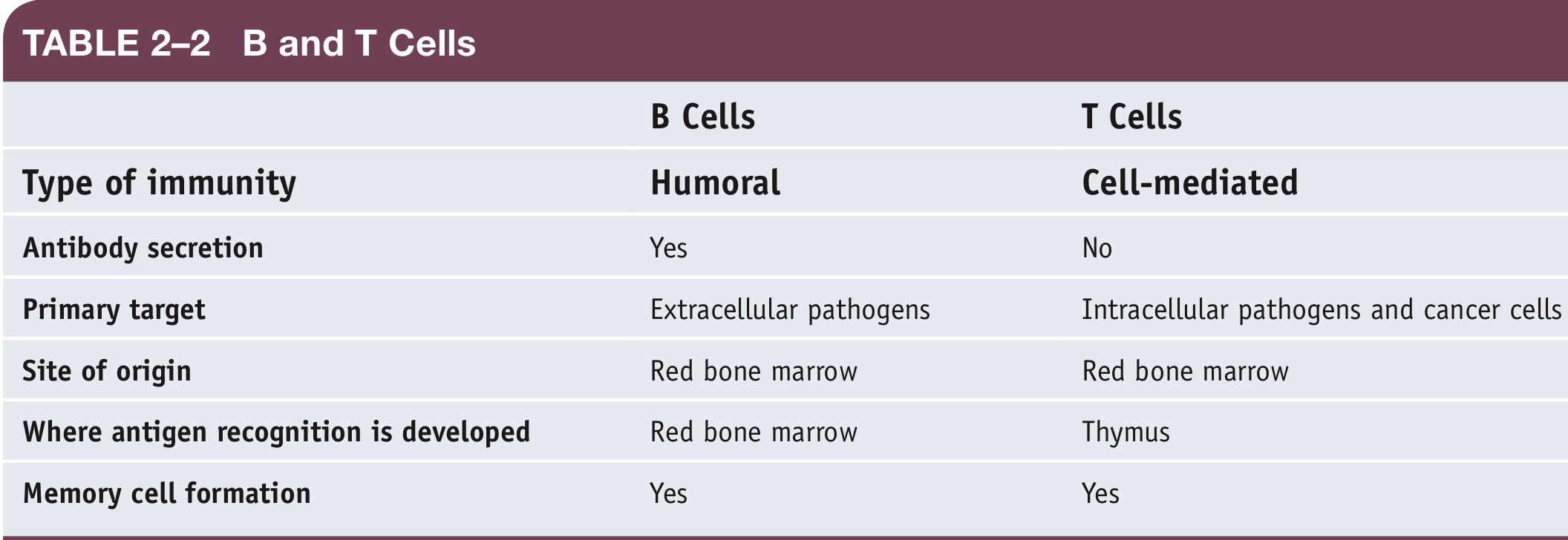
Summary of B and T Cells
\
29
New cards
Does immune system function decline with advancing age?
* Yes. The declines leads to a greater risk for infection and decreased ability to fight infectious diseases.
* As humans age, the thymus atrophies, cause a sharp decrease in the number and type of T cells produced. There are fewer T cells capable of responding to new antigens. Therefore, when older people encounter a new antigen, the body is less able to recognize and defend against it.
* It takes longer for macrophages to destroy bacteria, viruses, cancer cells, and other antigens. The amount of antibody produced in response to an antigen and an antibody’s ability to attach to an antigen are reduced. Because of reduced antibody production, vaccines are less likely to produce immunity in older people.
* While vaccines do not work as well in older adults, vaccinations for diseases such as influenza, pneumonia, hepatitis B, tuberculosis, diphtheria, and tetanus have been found to reduce mortality in older adults and are still worthwhile.
* As humans age, the thymus atrophies, cause a sharp decrease in the number and type of T cells produced. There are fewer T cells capable of responding to new antigens. Therefore, when older people encounter a new antigen, the body is less able to recognize and defend against it.
* It takes longer for macrophages to destroy bacteria, viruses, cancer cells, and other antigens. The amount of antibody produced in response to an antigen and an antibody’s ability to attach to an antigen are reduced. Because of reduced antibody production, vaccines are less likely to produce immunity in older people.
* While vaccines do not work as well in older adults, vaccinations for diseases such as influenza, pneumonia, hepatitis B, tuberculosis, diphtheria, and tetanus have been found to reduce mortality in older adults and are still worthwhile.
30
New cards
What tests detect the presence of antibodies to a specific pathogen or detect the presence of antigen from specific pathogen?
* Agglutination reactions of antigen and antibody detect bacterial (streptococci that causes strep throat) and viral (mononucleosis, measles, mumps, influenza) diseases and are used in blood typing.
* Enzyme immunoassay (EIA) uses an enzyme to label either the antibody or antigen. One of the most widely used EIA methods for detection of infectious diseases is the enzyme-linked immunosorbent assay (ELISA).
* Western blot detects the presence of antibodies in patient serum.
* Fluorescent antibody techniques use an antibody labeled with a fluorescent molecule to detect its antigen and are used to detect a wide variety of microorganisms.
* Flow cytometry identifies and counts cells that have a particular antigen. An interesting modification of a flow cytometer is the fluorescent-activated cell sorter (FACS). FACS is used to count helper T cells to follow the progression of HIV/AIDS.
* C-reactive protein and erythrocyte sedimentation tests measure general levels of inflammation in the body.
* Enzyme immunoassay (EIA) uses an enzyme to label either the antibody or antigen. One of the most widely used EIA methods for detection of infectious diseases is the enzyme-linked immunosorbent assay (ELISA).
* Western blot detects the presence of antibodies in patient serum.
* Fluorescent antibody techniques use an antibody labeled with a fluorescent molecule to detect its antigen and are used to detect a wide variety of microorganisms.
* Flow cytometry identifies and counts cells that have a particular antigen. An interesting modification of a flow cytometer is the fluorescent-activated cell sorter (FACS). FACS is used to count helper T cells to follow the progression of HIV/AIDS.
* C-reactive protein and erythrocyte sedimentation tests measure general levels of inflammation in the body.
31
New cards
tolerance
* the lack of response to the body’s own cells
* when tolerance fails, an autoimmune disease may result
* when tolerance fails, an autoimmune disease may result
32
New cards
Autoimmunity
* occurs when individuals develop antibodies called autoantibodies to their own tissues or self antigens
* also have autoreactive T cells
* no known cause, so cannot be prevented. But early diagnosis and treatment may prevent serious complications and greatly improve the patients quality of life
* also have autoreactive T cells
* no known cause, so cannot be prevented. But early diagnosis and treatment may prevent serious complications and greatly improve the patients quality of life
33
New cards
autoantibodies
* antibodies to your own tissues or self antigens
34
New cards
Lupus
* a chronic and relapsing autoimmune disease that can affect various parts of the body, including the skin, joints, heart lungs, blood, kidneys, and brain
* idiopathic, but environmental and genetic factors may play a role
* Environmental factors: infections, sulfa antibiotics and penicillin, ultraviolet light, stress, certain drugs, and hormones
* Genetic factors play a role: 10% of lupus patients have a parent or sibling with lupus. 5% of children develop lupus if their mother has lupus.
* incurable, but most live a normal lifespan. However, 10%-15% die prematurely
* Estrogen may influence the progression of lupus
* 1.5 mil Americans and 5 mil worldwide have a form of lupus. 90% of lupus patients are women, and diagnosis usually occurs between the ages of 15 and 44
* idiopathic, but environmental and genetic factors may play a role
* Environmental factors: infections, sulfa antibiotics and penicillin, ultraviolet light, stress, certain drugs, and hormones
* Genetic factors play a role: 10% of lupus patients have a parent or sibling with lupus. 5% of children develop lupus if their mother has lupus.
* incurable, but most live a normal lifespan. However, 10%-15% die prematurely
* Estrogen may influence the progression of lupus
* 1.5 mil Americans and 5 mil worldwide have a form of lupus. 90% of lupus patients are women, and diagnosis usually occurs between the ages of 15 and 44
35
New cards
What are the different types of lupus?
There are 4 types:
* Systemic (systemic lupus erythematous ‘SLE’) : 70% of people w/ lupus have this type. About half of SLE patients develop severe disease of the heart, lungs, kidneys, or brain. Common signs and symptoms; fatigue, arthritis, fever, butterfly rash across cheeks and nose, photosensitivity, mouth/nose ulcers, and Raynaud’s phenomenon.
* Cutaneous/discoid lupus: Affects only the skin and accounts for 10% of all lupus cases. There are many rashes and lesions causes by this but the most common rash is raised, scaly, and red, but no itchy. The rashes are circular. People may also have a butterfly rash across the cheeks and nose. 10% of people w/ cutaneous will develop SLE
* drug-induced/Drug-induced lupus erythematosus (DILE): can be brought on by more than 70 different prescription drugs and accounts for about 10% of all lupus cases. The signs and symptoms of DILE are similar to those of SLE; however, DILE rarely affects organs. The medications known to cause drug-induced lupus include hydralazine (used to treat high blood pressure) and procainamide and quinidine (used to treat irregular heart rhythm). Certain drugs entail a high risk, leading to DILE in 5–20% of people who use the drug for 1–2 years. The risk of developing DILE is less than 1% for most other drugs. Signs and symptoms disappear within days to months after discontinuing the drug.
* neonatal: acquired from maternal autoantibodies that affect the skin, heart, and blood of the fetus and newborn. It is characterized by a rash that appears within several weeks of life and persists about 6 months before disappearing.
* Systemic (systemic lupus erythematous ‘SLE’) : 70% of people w/ lupus have this type. About half of SLE patients develop severe disease of the heart, lungs, kidneys, or brain. Common signs and symptoms; fatigue, arthritis, fever, butterfly rash across cheeks and nose, photosensitivity, mouth/nose ulcers, and Raynaud’s phenomenon.
* Cutaneous/discoid lupus: Affects only the skin and accounts for 10% of all lupus cases. There are many rashes and lesions causes by this but the most common rash is raised, scaly, and red, but no itchy. The rashes are circular. People may also have a butterfly rash across the cheeks and nose. 10% of people w/ cutaneous will develop SLE
* drug-induced/Drug-induced lupus erythematosus (DILE): can be brought on by more than 70 different prescription drugs and accounts for about 10% of all lupus cases. The signs and symptoms of DILE are similar to those of SLE; however, DILE rarely affects organs. The medications known to cause drug-induced lupus include hydralazine (used to treat high blood pressure) and procainamide and quinidine (used to treat irregular heart rhythm). Certain drugs entail a high risk, leading to DILE in 5–20% of people who use the drug for 1–2 years. The risk of developing DILE is less than 1% for most other drugs. Signs and symptoms disappear within days to months after discontinuing the drug.
* neonatal: acquired from maternal autoantibodies that affect the skin, heart, and blood of the fetus and newborn. It is characterized by a rash that appears within several weeks of life and persists about 6 months before disappearing.
36
New cards
How can lupus be diagnosed?
* Lupus is difficult to diagnose. No single test will confirm the diagnosis of lupus
* Start by conducting a thorough medical history and physical exam.
* A complete blood count, urinalysis, autoantibody testing, measurement of general levels of inflammation, and biopsy may be performed.
* Start by conducting a thorough medical history and physical exam.
* A complete blood count, urinalysis, autoantibody testing, measurement of general levels of inflammation, and biopsy may be performed.
37
New cards
What can someone diagnosed with lupus do to manage it?
* Lupus patients should limit exposure to sunlight, never use tobacco, prevent fatigue and stress, eat a healthful diet, exercise, and take care of fevers over 100°F promptly.
38
New cards
What medications can be taken to treat and control lupus?
* nonsteroidal anti-inflammatory drugs (NSAIDs),
* steroidal anti-inflammatory drugs (corticosteroids),
* antimalarial medications,
* immunosuppressive drugs,
* monoclonal antibodies to inhibit autoantibody and antibody production.
* steroidal anti-inflammatory drugs (corticosteroids),
* antimalarial medications,
* immunosuppressive drugs,
* monoclonal antibodies to inhibit autoantibody and antibody production.
39
New cards
Secondary Raynaud’s phenomenon
fingers turning white and/or blue when cold
40
New cards
Scleroderma
* a chronic autoimmune disease of the connective tissue
* 300,000 Americans and 2.5 mil worldwide have Scleroderma
* 4x more common in women than in men, and the average age of diagnosis is in the 40s
* Two major forms are recognized: localized scleroderma (LSc), and Systemic sclerosis scleroderma (SSc)
* The most common initial signs and symptoms of scleroderma are secondary Raynaud’s phenomenon, thickening and tightening of the skin of the fingers, and pain in two or more joints. Early symptoms can also include heartburn, difficulty swallowing, or shortness of breath.
* etiology is idiopathic, but immune, genetic, environmental, and hormonal factors play a role.
* May be linked to estrogen that’s why more common in women
* The immune system may stimulate fibroblasts to produce too much collagen, a tough connective-tissue protein.
* No environmental agent has been shown to cause scleroderma, yet research suggests that exposure to viral infections and certain chemicals may trigger scleroderma in those who are genetically predisposed.
* There is no cure and it is not preventable
* 300,000 Americans and 2.5 mil worldwide have Scleroderma
* 4x more common in women than in men, and the average age of diagnosis is in the 40s
* Two major forms are recognized: localized scleroderma (LSc), and Systemic sclerosis scleroderma (SSc)
* The most common initial signs and symptoms of scleroderma are secondary Raynaud’s phenomenon, thickening and tightening of the skin of the fingers, and pain in two or more joints. Early symptoms can also include heartburn, difficulty swallowing, or shortness of breath.
* etiology is idiopathic, but immune, genetic, environmental, and hormonal factors play a role.
* May be linked to estrogen that’s why more common in women
* The immune system may stimulate fibroblasts to produce too much collagen, a tough connective-tissue protein.
* No environmental agent has been shown to cause scleroderma, yet research suggests that exposure to viral infections and certain chemicals may trigger scleroderma in those who are genetically predisposed.
* There is no cure and it is not preventable
41
New cards
What are the 2 major forms of scleroderma?
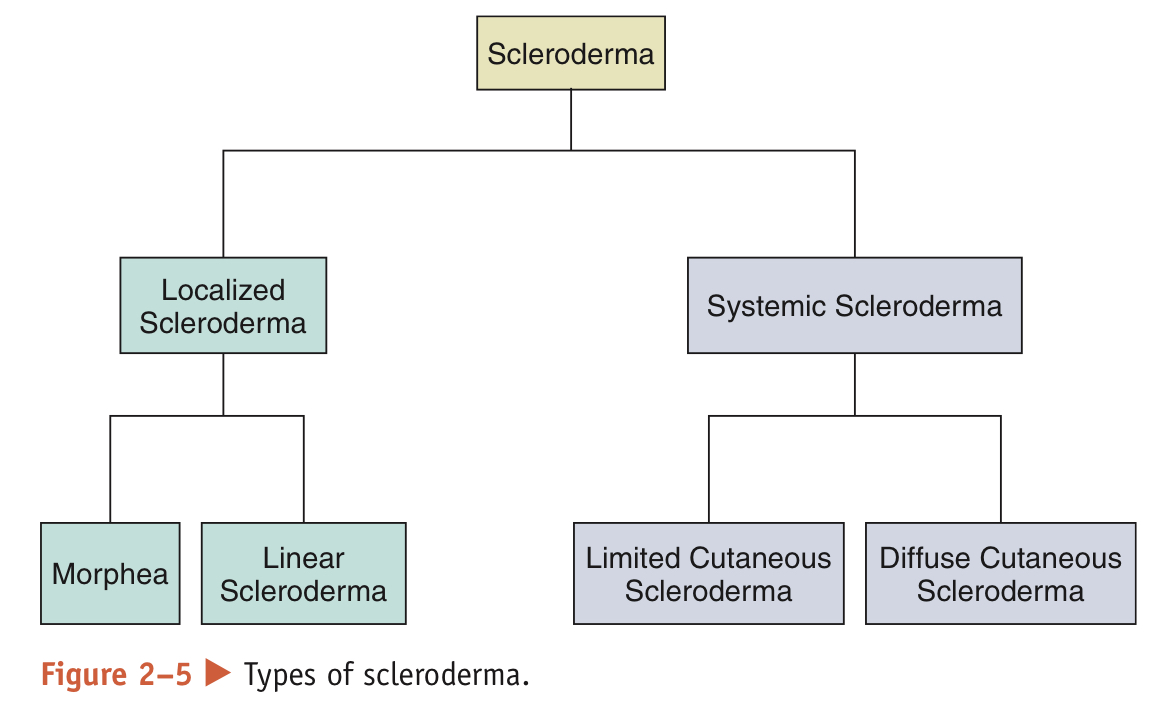
* Localized Scleroderma (LSc): More common and only affects the skin
* appears in form of waxy patches (morphea) or streaks on the skin (linear scleroderma)
* Tends to regress or stop progressing w/o treatment, but can be progressive and disfiguring, requiring treatment to control disease activity
* Patients w/ LSc have a normal lifespan
* Systemic Sclerosis Scleroderma (SSc): affects skin and internal organs
* Classified into 2 subjects based on extent of skin tightening:
* Limited cutaneous SSc (lcSSc): skin tightening is confined to the fingers, hands, and arms below the elbows, and may involve the feet and legs below the knees. 10-year survival rate: 71%
* Diffuse cutaneous SSc (dcSSc): skin tightening is confined to the fingers, hands, and arms below and above the elbows, feet, legs and knees, and trunk. 10-year survival rate: 21%
* Both lcSSc and dcSSc affect internal organs, but patients with dcSSc are at a greater risk for significant organ dysfunction.
* appears in form of waxy patches (morphea) or streaks on the skin (linear scleroderma)
* Tends to regress or stop progressing w/o treatment, but can be progressive and disfiguring, requiring treatment to control disease activity
* Patients w/ LSc have a normal lifespan
* Systemic Sclerosis Scleroderma (SSc): affects skin and internal organs
* Classified into 2 subjects based on extent of skin tightening:
* Limited cutaneous SSc (lcSSc): skin tightening is confined to the fingers, hands, and arms below the elbows, and may involve the feet and legs below the knees. 10-year survival rate: 71%
* Diffuse cutaneous SSc (dcSSc): skin tightening is confined to the fingers, hands, and arms below and above the elbows, feet, legs and knees, and trunk. 10-year survival rate: 21%
* Both lcSSc and dcSSc affect internal organs, but patients with dcSSc are at a greater risk for significant organ dysfunction.
42
New cards
How can you diagnose scleroderma?
* Diagnosis begins with a thorough medical history and physical exam.
* No single test will confirm the diagnosis of scleroderma.
* Autoantibody testing and biopsy may be performed.
* No single test will confirm the diagnosis of scleroderma.
* Autoantibody testing and biopsy may be performed.
43
New cards
What can Scleroderma patients do to help ease the challenges?
* Scleroderma patients should not use tobacco, should avoid exposure to cold and stress, exercise to help keep skin and joints flexible, eat small frequent meals, protect their skin, and moisturize the skin frequently.
44
New cards
How can you treat Scleroderma?
* several medications can treat and control scleroderma: anti-inflammatory drugs, immunosuppressive drugs, and vasodilators to treat Raynaud’s.
45
New cards
Sjögren’s Syndrome
* Sjögren’s syndrome is a chronic, slowly progressive autoimmune disease that affects the exocrine (moisture-producing) glands of the body
* 4 million americans have Sjögren’s syndrome. 90% are women and often affects them during their childbearing years, suggesting a link w/ estrogen
* Half of the cases are called primary Sjören’s syndrome. Other half is called secondary Sjögren’s syndrome because they occur w/ another diseases like
rheumatoid arthritis, lupus, or scleroderma.
* The hallmark symptoms of Sjögren’s syndrome are dry eyes and mouth. Sjögren’s may also cause complications in the kidneys, gastrointestinal system, blood vessels, lungs, liver, pancreas, and central nervous system.
* etiology is idiopathic, but genetic factors, hormones, and environmental triggers (viral infections) play a role. A first-degree relative with autoimmunity increases the risk for Sjögren’s syndrome sevenfold
* Often undiagnosed or misdiagnosed because its signs and symptoms mimic those of menopause, drug side effects, or diseases like lupus.
* 4 million americans have Sjögren’s syndrome. 90% are women and often affects them during their childbearing years, suggesting a link w/ estrogen
* Half of the cases are called primary Sjören’s syndrome. Other half is called secondary Sjögren’s syndrome because they occur w/ another diseases like
rheumatoid arthritis, lupus, or scleroderma.
* The hallmark symptoms of Sjögren’s syndrome are dry eyes and mouth. Sjögren’s may also cause complications in the kidneys, gastrointestinal system, blood vessels, lungs, liver, pancreas, and central nervous system.
* etiology is idiopathic, but genetic factors, hormones, and environmental triggers (viral infections) play a role. A first-degree relative with autoimmunity increases the risk for Sjögren’s syndrome sevenfold
* Often undiagnosed or misdiagnosed because its signs and symptoms mimic those of menopause, drug side effects, or diseases like lupus.
46
New cards
How can you diagnose Sjögren’s syndrome?
* Diagnosis begins with a thorough medical history and physical exam.
* No single test will confirm the diagnosis of Sjögren’s syndrome.
* Tear and saliva tests, autoantibody testing, and lip or salivary gland biopsy may be performed.
* No single test will confirm the diagnosis of Sjögren’s syndrome.
* Tear and saliva tests, autoantibody testing, and lip or salivary gland biopsy may be performed.
47
New cards
How can you treat Sjögren’s syndrome?
* No cure and cannot be prevented
* Several medications alleviate the symptoms:
* over-the-counter and prescription medications for dry eyes and dry mouth,
* NSAIDs,
* immunosuppressive drugs,
* vasodilators to treat Raynaud’s,
* and other symptomatic treatments to treat heartburn and high blood pressure and to improve breathing.
* Several medications alleviate the symptoms:
* over-the-counter and prescription medications for dry eyes and dry mouth,
* NSAIDs,
* immunosuppressive drugs,
* vasodilators to treat Raynaud’s,
* and other symptomatic treatments to treat heartburn and high blood pressure and to improve breathing.
48
New cards
Allergy (hypersensitivity)
an extreme immune response to a harmless antigen
49
New cards
allergen
the normally harmless antigen that causes an allergic response
(ie) ragweed, grass pollen, mold, bee venom, latex, etc
(ie) ragweed, grass pollen, mold, bee venom, latex, etc
50
New cards
How many and what are the different types of Hypersensitivity?
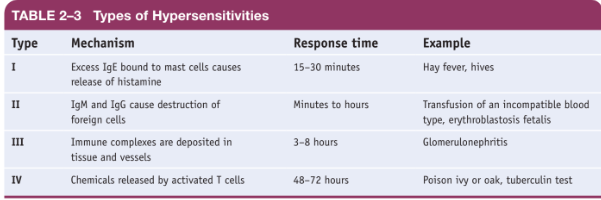
There are several different types of hypersensitivity:
* immediate (or Type I),
* cytotoxic (or Type II),
* immune-complex (or Type III),
* and delayed (or Type IV).
* immediate (or Type I),
* cytotoxic (or Type II),
* immune-complex (or Type III),
* and delayed (or Type IV).
51
New cards
Type 1 Hypersensitivity
* Type I is the most common type of
allergy.
* Type I is a local allergy, occurring rapidly
where the allergen encounters the body.
* The Type I reaction is triggered by IgE, the immunoglobulin that responds to the presence of allergens.
* The allergy problems arise because the IgE also binds to mast cells and induces them to release histamine and other potent chemicals responsible for allergy symptoms. Histamine dilates the blood vessels, causing them to leak plasma into the tissues. This tissue fluid causes edema, or swelling, where the allergen was encountered.
* Edema localized in the nasal passages results in the familiar congestion and irritation of hay fever. Edema in the skin causes the welts and itching of hives.
* Inhaled allergens can cause signs and symptoms of asthma. Antihistamines inhibit the effects of histamine. Decongestants do not affect histamine, but they do relieve symptoms.
allergy.
* Type I is a local allergy, occurring rapidly
where the allergen encounters the body.
* The Type I reaction is triggered by IgE, the immunoglobulin that responds to the presence of allergens.
* The allergy problems arise because the IgE also binds to mast cells and induces them to release histamine and other potent chemicals responsible for allergy symptoms. Histamine dilates the blood vessels, causing them to leak plasma into the tissues. This tissue fluid causes edema, or swelling, where the allergen was encountered.
* Edema localized in the nasal passages results in the familiar congestion and irritation of hay fever. Edema in the skin causes the welts and itching of hives.
* Inhaled allergens can cause signs and symptoms of asthma. Antihistamines inhibit the effects of histamine. Decongestants do not affect histamine, but they do relieve symptoms.
52
New cards
Anaphylaxis
* Immediate hypersensitivity may cause a systemic, acute allergic response (anaphylaxis) that may be life-threatening
* Allergens that cause anaphylaxis are found in foods such as peanuts, in latex, in some medications such as penicillin, and in the venom of stinging insects such as wasps and bees.
* Signs and symptoms of anaphylaxis include a sudden drop in blood pressure, narrowing of the airways, rapid and weak pulse, hives, and nausea and vomiting.
* Antihistamines, corticosteroids, and epinephrine are used to treat anaphylaxis.
* Epinephrine by injection is the treatment of choice for anaphylaxis because it quickly begins working to reverse symptoms of anaphylaxis.
* Epinephrine constricts blood vessels to increase blood pressure, relaxes smooth muscles in the lungs to reduce wheezing and improve breathing, increases heart rate, and works to reduce hives and swelling that may occur around the face and lips.
* Allergens that cause anaphylaxis are found in foods such as peanuts, in latex, in some medications such as penicillin, and in the venom of stinging insects such as wasps and bees.
* Signs and symptoms of anaphylaxis include a sudden drop in blood pressure, narrowing of the airways, rapid and weak pulse, hives, and nausea and vomiting.
* Antihistamines, corticosteroids, and epinephrine are used to treat anaphylaxis.
* Epinephrine by injection is the treatment of choice for anaphylaxis because it quickly begins working to reverse symptoms of anaphylaxis.
* Epinephrine constricts blood vessels to increase blood pressure, relaxes smooth muscles in the lungs to reduce wheezing and improve breathing, increases heart rate, and works to reduce hives and swelling that may occur around the face and lips.
53
New cards
Why is an epinephrine-injection device a prescription medication?
\
54
New cards
Why is an epinephrine-injection device injected into the thigh muscle?
55
New cards
Type II (Cytotoxic) Hypersensitivity
* Type II hypersensitivity is called cytotoxic
because IgM or IgG causes the destruction of
cells.
* An example of cytotoxic hypersensitivity is
the response to an incompatible blood transfu-
sion.
* A person with type A blood has A antigens
on their red cells and antibodies against type B
blood in their serum. If such a person receives a
type B transfusion, his or her antibodies interact
with the antigens on the transfused red blood
cells. The red blood cells agglutinate, or clump
together, and lyse (rupture). .
because IgM or IgG causes the destruction of
cells.
* An example of cytotoxic hypersensitivity is
the response to an incompatible blood transfu-
sion.
* A person with type A blood has A antigens
on their red cells and antibodies against type B
blood in their serum. If such a person receives a
type B transfusion, his or her antibodies interact
with the antigens on the transfused red blood
cells. The red blood cells agglutinate, or clump
together, and lyse (rupture). .
56
New cards
Type III (immune-complex) hypersensitivity
* involves antigens combining with many antibodies in the blood, forming a soluble mass of antigens and antibodies known as immune complexes.
* These immune complexes deposit in tissues and blood vessels where they trigger inflammation and tissue destruction.
* After a streptococcal infection, immune complexes composed of streptococcal antigens and antibodies might form in the kidneys, where they cause the inflammatory disease glomerulonephritis.
* These immune complexes deposit in tissues and blood vessels where they trigger inflammation and tissue destruction.
* After a streptococcal infection, immune complexes composed of streptococcal antigens and antibodies might form in the kidneys, where they cause the inflammatory disease glomerulonephritis.
57
New cards
Type IV (delayed) Hypersensitivity
* Type IV hypersensitivities are called delayed hypersensitivities because they take time to develop following exposure to an allergen.
* The delay occurs because the allergy is due to the action of T cells, which require time to recognize the allergen, reproduce and differentiate, and bring about the allergy symptoms.
* Delayed hypersensitivity includes the skin reaction to poison ivy or poison oak, contact dermatitis from wearing latex gloves, and the tuberculosis skin test.
* Antihistamines are not effective against delayed hypersensitivity, but corticosteroids provide some relief.
* The delay occurs because the allergy is due to the action of T cells, which require time to recognize the allergen, reproduce and differentiate, and bring about the allergy symptoms.
* Delayed hypersensitivity includes the skin reaction to poison ivy or poison oak, contact dermatitis from wearing latex gloves, and the tuberculosis skin test.
* Antihistamines are not effective against delayed hypersensitivity, but corticosteroids provide some relief.
58
New cards
Allergy Testing
* A variety of tests can diagnose allergies.
* A commonly used allergy test is the skin test, in which a small amount of the suspected allergen is placed on or below the skin to assess the skin’s response to the allergen.
* Types of skin tests include the skin prick test, intradermal test, and skin patch test.
* Alternatively, blood tests can detect allergen-specific IgE.
* A commonly used allergy test is the skin test, in which a small amount of the suspected allergen is placed on or below the skin to assess the skin’s response to the allergen.
* Types of skin tests include the skin prick test, intradermal test, and skin patch test.
* Alternatively, blood tests can detect allergen-specific IgE.
59
New cards
Allergy Treatment
* Allergy symptoms are treated with medicines,
although prevention is preferable.
* People should avoid known allergens, or they may be able to take allergy shots that desensitize them to the allergen.
* During desensitization, allergens are
administered in small amounts, then in gradually increased doses. These allergy shots induce
the production of IgG in the blood, which coats
the allergen and blocks it from binding to IgE
in the tissues.
although prevention is preferable.
* People should avoid known allergens, or they may be able to take allergy shots that desensitize them to the allergen.
* During desensitization, allergens are
administered in small amounts, then in gradually increased doses. These allergy shots induce
the production of IgG in the blood, which coats
the allergen and blocks it from binding to IgE
in the tissues.
60
New cards
Acquired Immunodeficiency Syndrome (AIDS)
a disease of the immune system characterized by a reduction in the number of helper T cells (CD4 cells) and an increased susceptibility to opportunistic infections and certain cancers.
61
New cards
Human Immunodeficiency Virus (HIV)
* a virus that attacks the body's immune system.
* Can lead to AIDS
* Risk factors for HIV/AIDS include:
* having unprotected sex,
* having multiple sex partners,
* having another sexually transmitted infection,
* IV drug use,
* being uncircumcised,
* and being born to an infected mother.
* Can lead to AIDS
* Risk factors for HIV/AIDS include:
* having unprotected sex,
* having multiple sex partners,
* having another sexually transmitted infection,
* IV drug use,
* being uncircumcised,
* and being born to an infected mother.
62
New cards
What are the different stages of HIV infection?
1. Primary HIV infection: follows exposure to HIV, lasts a few weeks, and is accompanied by a short flulike illness
2. Clinically Asymptomatic Stage: patients don’t have symptoms, but they can transmit the infection, and HIV continues to multiply, infecting and killing helper T cells. Lasts an average of 10 years
3. Symptomatic HIV: when an HIV-infected patient experiences symptoms but has not developed AIDS. Signs and symptoms can persist for several years and include diarrhea, fever or night sweats, fatigue or joint pain, oral infections, enlarged lymph nodes, and skin problems.
4. Progression from HIV to AIDS: At this stage the patient has one of the AIDS indicator diseases and a helper T cell count of less than 200. AIDS indicator diseases are infections that do not normally occur in a person protected by a healthy immune system.
1. Examples of these infections include pneumonia caused by the fungus Pneumocystis jirovecii.
63
New cards
What is the cause of AIDS?
The cause of AIDS is the human immunodeficiency virus, a retrovirus that carries its genetic information as RNA rather than DNA
64
New cards
How is HIV transmitted?
* Transmitted via contaminated body fluids:
* blood
* semen
* vaginal secretions
* breast milk
* Therefore, HIV is transmitted by unprotected anal/oral/vaginal intercourse, during birth, through breastfeeding, and by sharing needles
* blood
* semen
* vaginal secretions
* breast milk
* Therefore, HIV is transmitted by unprotected anal/oral/vaginal intercourse, during birth, through breastfeeding, and by sharing needles
65
New cards
How is HIV diagnosed?
HIV is diagnosed using ELISA to detect HIV antibodies in the blood. Most people produce antibodies against HIV within 3 months of being infected. A positive ELISA is repeated and the result confirmed using the Western blot.
66
New cards
How is HIV treated?
* HIV is treated through Antiretroviral therapy (ART).
* ART controls replication of the virus and slows the progression of HIV-related disease.
* ART includes several types of drugs:
* reverse transcriptase inhibitors interfere with the conversion of HIV RNA to DNA;
* protease inhibitors stop the assembly of new HIV viruses;
* fusion inhibitors prevent the fusion of HIV to helper T cells;
* entry inhibitors block HIV from entering helper T cells;
* and integrase inhibitors interfere with HIV inserting its genetic material into human cells.
* ART does NOT cure HIV
* ART controls replication of the virus and slows the progression of HIV-related disease.
* ART includes several types of drugs:
* reverse transcriptase inhibitors interfere with the conversion of HIV RNA to DNA;
* protease inhibitors stop the assembly of new HIV viruses;
* fusion inhibitors prevent the fusion of HIV to helper T cells;
* entry inhibitors block HIV from entering helper T cells;
* and integrase inhibitors interfere with HIV inserting its genetic material into human cells.
* ART does NOT cure HIV
67
New cards
How can I prevent HIV?
The WHO suggests the following for HIV prevention:
* Abstinence
* Monogamy
* Condom use
* Testing and counseling for HIV
* Male circumcision
* Sterile needles and syringes for each injection for IV drug use
* ART therapy
* ➢ Preexposure prophylaxis for an HIV-negative partner
* ➢ Postexposure prophylaxis for accidental exposure
* ➢ To prevent mother-to-child transmission
* Abstinence
* Monogamy
* Condom use
* Testing and counseling for HIV
* Male circumcision
* Sterile needles and syringes for each injection for IV drug use
* ART therapy
* ➢ Preexposure prophylaxis for an HIV-negative partner
* ➢ Postexposure prophylaxis for accidental exposure
* ➢ To prevent mother-to-child transmission
68
New cards
Is the HIV preventative drug a cure for HIV and AIDS?
No
69
New cards
Is the HIV preventative drug a substitute for safer sex practices?
Yes.
70
New cards
Hodgkin’s Lymphoma (disease)
* a cancer of the immune system
* most frequently diagnosed between the ages of 15 and 40 and after 55
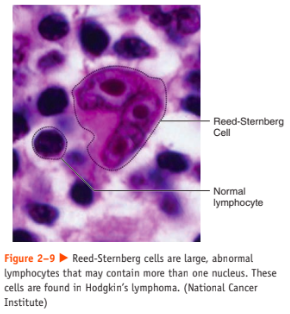
* marked by the presence of a type of cell called the Reed-Sternberg cell
* includes a wide variety of diverse subtypes, each of which may have a distinct treatment and prognosis.
* Risk factors include:
* certain viral infections (Epstein-Barr and HIV),
* a weakened immune system,
* and a family history of Hodgkin’s lymphoma.
* Signs and symptoms include:
* painless swelling of lymph nodes (neck, armpit, or groin),
* fatigue,
* unexplained fever,
* soaking night sweats,
* itchy skin,
* coughing,
* trouble breathing,
* chest pain
* unexplained weight loss
* etiology is idiopathic
* most frequently diagnosed between the ages of 15 and 40 and after 55
* marked by the presence of a type of cell called the Reed-Sternberg cell
* includes a wide variety of diverse subtypes, each of which may have a distinct treatment and prognosis.
* Risk factors include:
* certain viral infections (Epstein-Barr and HIV),
* a weakened immune system,
* and a family history of Hodgkin’s lymphoma.
* Signs and symptoms include:
* painless swelling of lymph nodes (neck, armpit, or groin),
* fatigue,
* unexplained fever,
* soaking night sweats,
* itchy skin,
* coughing,
* trouble breathing,
* chest pain
* unexplained weight loss
* etiology is idiopathic
71
New cards
How do you diagnose Hodgkin’s Lymphoma?
Hodgkin’s lymphoma diagnosis is based on a physical exam, complete blood count, and biopsy to confirm the presence of Reed-Sternberg cells. Imaging tests and a bone marrow biopsy may be used to stage the disease.
72
New cards
How is Hodgkin’s Lymphoma treated?
Hodgkin’s lymphoma may be treated with:
* chemotherapy,
* radiation therapy,
* and bone marrow or stem cell transplant.
Survival of Hodgkin’s lymphoma is related to the extent of disease at diagnosis. The 5-year survival for all stages of the disease combined is estimated at 65–90%. Unfortunately, Hodgkin’s lymphoma cannot be prevented.
* chemotherapy,
* radiation therapy,
* and bone marrow or stem cell transplant.
Survival of Hodgkin’s lymphoma is related to the extent of disease at diagnosis. The 5-year survival for all stages of the disease combined is estimated at 65–90%. Unfortunately, Hodgkin’s lymphoma cannot be prevented.
73
New cards
Non-Hodgkin’s lymphoma
* cancer of the lymphocytes.
* most frequently diagnosed in adults age 60 and older.
* Like Hodgkin’s disease, non-Hodgkin’s lymphoma includes a wide variety of subtypes.
* Risk factors for non-Hodgkin’s lymphoma include:
* a weakened immune system and certain infections (human T-cell lymphotropic virus,
Epstein-Barr virus, Helicobacter pylori, hepati-
tis C, HIV)
* Signs and symptoms include:
* painless swelling of lymph nodes (neck, armpit, or groin),
* fatigue,
* pain,
* unexplained fever,
* soaking night sweats,
* itchy skin
* coughing
* trouble breathing,
* chest pain,
* swelling or a feeling of fullness in the abdomen,
* and unexplained weight loss.
* The etiology of non-Hodgkin’s lymphoma is idiopathic.
* most frequently diagnosed in adults age 60 and older.
* Like Hodgkin’s disease, non-Hodgkin’s lymphoma includes a wide variety of subtypes.
* Risk factors for non-Hodgkin’s lymphoma include:
* a weakened immune system and certain infections (human T-cell lymphotropic virus,
Epstein-Barr virus, Helicobacter pylori, hepati-
tis C, HIV)
* Signs and symptoms include:
* painless swelling of lymph nodes (neck, armpit, or groin),
* fatigue,
* pain,
* unexplained fever,
* soaking night sweats,
* itchy skin
* coughing
* trouble breathing,
* chest pain,
* swelling or a feeling of fullness in the abdomen,
* and unexplained weight loss.
* The etiology of non-Hodgkin’s lymphoma is idiopathic.
74
New cards
How can you diagnose Non-Hodgkin’s Lymphoma?
* Diagnosis of non-Hodgkin’s lymphoma is based on a physical exam, complete blood count, lactate dehydrogenase test (tests for tissue damage), and biopsy. Imaging tests, bone marrow biopsy, and a lumbar puncture are used to stage the disease.
75
New cards
How can you treat Non-Hodgkin’s Lymphoma
* Non-Hodgkin’s lymphoma that is slow-growing may not require treatment for years. In these cases
“watch and wait” may be a treatment option.
* Treatment may include:
* radiation therapy,
* chemotherapy,
* anti-inflammatory medication,
* stem cell transplant,
* and monoclonal antibody therapy to tag cancer cells for destruction.
* Prognosis varies and depends on the stage of
the cancer, the subtype, patient age, and overall health of the patient.
* Screening and early diagnosis are important because non-Hodgkin’s lymphoma cannot be prevented.
“watch and wait” may be a treatment option.
* Treatment may include:
* radiation therapy,
* chemotherapy,
* anti-inflammatory medication,
* stem cell transplant,
* and monoclonal antibody therapy to tag cancer cells for destruction.
* Prognosis varies and depends on the stage of
the cancer, the subtype, patient age, and overall health of the patient.
* Screening and early diagnosis are important because non-Hodgkin’s lymphoma cannot be prevented.
76
New cards
Tom was not vaccinated against the rubella virus and is concerned he may have contracted measles. His physician takes a blood sample and sends it to a lab to measure rubella antibody levels. The results show an elevated level of IgM antibodies to the rubella virus but very few IgG antibodies to the virus. Did Tom contract measles? How do you know? Did Tom contract the rubella virus recently? How do you know?
\
77
New cards
A crime scene investigator finds a body of a woman and discovers animal bites on the victim’s body. The investigator examines the bites and sees they are not inflamed. Did the animal bites happen before or after the woman died?
\
78
New cards
Some people with decreased IgA exhibit recurrent sinus and respiratory infections. Why?
\
79
New cards
Explain why HIV’s attack on helper T cells
devastates the entire immune system.
devastates the entire immune system.
80
New cards
81
New cards
1. The body’s ability to resist disease is
____________________.
a. specific immunity
b. nonspecific immunity
c. immunity
d. antigen
e. tolerance
c. immunity
82
New cards
A type of leukocyte that recognizes and eliminates viral infected cells and cancer cells is ____________________.
a. helper T cell
b. cytotoxic T cell
c. natural killer cell
d. memory cell
e. plasma cell
a. helper T cell
b. cytotoxic T cell
c. natural killer cell
d. memory cell
e. plasma cell
83
New cards
Defense against extracellular antigens via antibodies is called ____________________.
a. humoral immunity
b. cell-mediated immunity
c. plasma cells
d. tolerance
e. suppression
a. humoral immunity
b. cell-mediated immunity
c. plasma cells
d. tolerance
e. suppression
84
New cards
Nonspecific immunity includes all the following except
a. fever
b. cell-mediated immunity
c. phagocytosis
d. interferons
a. fever
b. cell-mediated immunity
c. phagocytosis
d. interferons
5. Which is any foreign substance that
when introduced into the body is
recognized as nonself and activates
the immune system?
a. antigen
b. antibody
c. antibiotic
d. fever
85
New cards
Which cells secrete antibodies during the immune response?
a. helper T cells
b. memory cells
c. plasma cells
d. cytotoxic T cells
a. helper T cells
b. memory cells
c. plasma cells
d. cytotoxic T cells
The response to poison ivy is an example of which type of hypersensitivity?
a. I
b. II
c. III
d. IV
5. Which antibody is produced in excess during an allergic response? a. IgE b. IgG c. IgM d. IgA
a. I
b. II
c. III
d. IV
5. Which antibody is produced in excess during an allergic response? a. IgE b. IgG c. IgM d. IgA
86
New cards
87
New cards