CHEM 106 FINAL
0.0(0)
0.0(0)
Card Sorting
1/163
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
164 Terms
1
New cards
The dramatic temperature changes on the moon between day and night are not seen on the earth because of what property of water?
high heat capacity
2
New cards
Heat of vaporization is the amount of heat required to
evaporate a liquid
3
New cards
Soft water contains relatively large concentrations of
sodium ions
4
New cards
The EPA drinking water standard for arsenic is 0.030mg/L. What is this concentration in ppm?
0\.030
5
New cards
Fluorides are added to water to
prevent tooth decay
6
New cards
Which of the following statements about bottled drinking water is true?
bottled mineral water often contains more dissolved ions than tap water
7
New cards
Advanced treatment of wastewater can include any of the following except
sand and gravel filtration
8
New cards
consumption of water with high levels of nitrates is particularly hazardous to
infants
9
New cards
which part of the water supply is most isolated from the purifying capacity of the water cycle
groundwater
10
New cards
Water's unique properties, including its high heat capacity, high density, and a solid phase that is less dense than its liquid phase, can best be attributed to
the polarity of the molecule and hydrogen bonding between the molecules
11
New cards
The SI unit of energy is
Joule
12
New cards
When the overall equation for the photosynthesis process is written and balanced, how many molecules of water are required to make one glucose molecule, C6H12O6?
6
13
New cards
How many joules of energy will a stock tank heater rated at 400 watts use in a 48 hour period?
400 x 48 x 3600
14
New cards
All of the following examples are classified as potential energy EXCEPT
energy of a moving object
15
New cards
Chemical reactions that release heat to the surroundings are endothermic reactions.
false
16
New cards
According to kinetic-molecular theory, reactions occur more rapidly at higher temperatures because
molecules collide more frequently at higher temperatures
molecules move faster at higher temperatures
molecules have more energy at higher temperatures
molecules move faster at higher temperatures
molecules have more energy at higher temperatures
17
New cards
The decomposition of 2 moles of water to hydrogen and oxygen requires 137 kcal of energy. The reaction of hydrogen and oxygen to form 2 moles of water
releases 137 kcal
18
New cards
Which of the following does NOT result in an increase in entropy?
student cleans his dorm room
19
New cards
which of the following is not made from petroleum
\
Tires
plastics
detergents
perfumes
\
Tires
plastics
detergents
perfumes
Tires
20
New cards
Which problem do all power plants have in common?
Thermal Pollution
21
New cards
When coal burns
chemical energy is converted to heat energy
22
New cards
Which of the following is NOT a problem associated with nuclear power plants?
air pollution
23
New cards
Biomass may be burned directly or chemically converted to a more convenient form (liquid or gas). Which of the following statements is true?
The conversion of biomass to a more convenient form decreases the overall efficiency of the energy conversion.
24
New cards
Which pollutants are reduced in concentration by catalytic converters?
Nitrogen oxide (NO) and carbon monoxide (CO)
25
New cards
The presence of nitrogen dioxide in smog is most easily recognized by its
color
26
New cards
An atmospheric inversion is a phenomenon that may have disastrous effects with respect to air pollution. An atmospheric inversion occurs when a
lower layer of cool air is trapped by an upper layer of warmer air
27
New cards
The most abundant fossil fuel in the United States is
coal
28
New cards
Which of the following is a product of photosynthesis?
O2
29
New cards
A student mixes compound A with compound B and notices that a chemical reaction occurs. The temperature of the mixture decreases. The reaction is ____________.
Endothermic
30
New cards
Approximately what percentage of the energy used to support our current lifestyle comes from fossil fuels?
over 85%
31
New cards
How much heat, in kilojoules, is released when 12.3 grams of H2(g) reacts with plenty of O2(g) to form steam (H2O(g)) according to the following equation?
2H2(g) + O2(g) ⟶ 2H2O(g) + 483.60kJ
2H2(g) + O2(g) ⟶ 2H2O(g) + 483.60kJ
1\.48 x 10^3 kJ
32
New cards
If we shine a green laser on a sheet of metal, we are able to quantify that electrons are emitted from the metal's surface. Which other colored lasers would cause the metal to emit electrons?
purple and blue
33
New cards
Based on the temperature change observed in the bottle, what type of reaction (in terms of energy change) was performed?
exothermic
34
New cards
As the rate of carbon dioxide emissions increases, the effects of global warming also increase.
true
35
New cards
What molecule(s) is/are responsible for the Greenhouse Effect, which traps energy (in the form of heat) in the atmosphere and sends it back towards the earth?
Methane
CO2
water vapor
Nitrous oxide
CO2
water vapor
Nitrous oxide
36
New cards
Refer to the chemical equation for the combustion of octane (be sure to balance the equation) and the given useful information:
C8H18 + O2 ⟶ CO2 + H2O
1 pound = 453 grams
Density of octane = 0.070g/cm3
1 gallon = 3.79 liters
C8H18 + O2 ⟶ CO2 + H2O
1 pound = 453 grams
Density of octane = 0.070g/cm3
1 gallon = 3.79 liters
.37 kilograms of octane must burn to emit 25 pounds of carbon dioxide into the atmosphere.
This is equivalent to 1.4 gallons of octane.
This is equivalent to 1.4 gallons of octane.
37
New cards
All of the following are properties of hydrocarbons EXCEPT
\
They burn readily
their boiling points increase as the number of carbons increases
they are good solvents for nonpolar substances
they have a higher density than water
\
They burn readily
their boiling points increase as the number of carbons increases
they are good solvents for nonpolar substances
they have a higher density than water
they have a higher density than water
38
New cards
A compound containing only carbon and hydrogen and which has only single bonds between atoms is classified as an
alkane
39
New cards
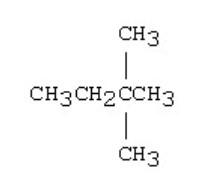
The condensed structural formula for the molecule below is
\n CH3CH2CH2(CH3)2CH3
40
New cards
Which of the following is an alkene?
\
benzene
propene
acetylene
heptane
\
benzene
propene
acetylene
heptane
propene
41
New cards
The name of the three carbon alkane is ___________.
\
\
propane
42
New cards
A compound that has at least one double bond is aromatic.
false
43
New cards
What is the name of the compound with a formula CH3CH2CH2CH3?
butane
44
New cards
Organic compounds that contain a benzene ring or possess certain properties similar to those of benzene are called _______ compounds.
aromatic
45
New cards
Which of the following options completes the sentences correctly?
The general formula RCOOH belongs to the __________ family, the general formula ROH belongs to the __________ family, and the general formula RNH2 belongs to the __________ family.
The general formula RCOOH belongs to the __________ family, the general formula ROH belongs to the __________ family, and the general formula RNH2 belongs to the __________ family.
carboxylic acid, alcohol, amine
46
New cards
All of the following contain more than one hydroxyl group EXCEPT
methanol
47
New cards
Which statement about Methane is incorrect?
\
It is the most simple alkane.
It is the largest component of natural gas.
It has an odor similar to that of a sewer.
It is highly flammable.
\
It is the most simple alkane.
It is the largest component of natural gas.
It has an odor similar to that of a sewer.
It is highly flammable.
It has an odor similar to that of a sewer.
48
New cards
Which of the following is the most likely to ignite slowly and produce a large amount of smoke when burned?
Octane, C8H18
49
New cards
Which of the compounds below is likely to form a metallic mirror on test tube walls when added to a mixture of aqueous silver nitrate and sodium hydroxide?
\
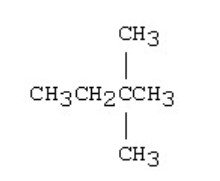
50
New cards
Which of the following is the correct reason that a Tollens' test can distinguish between aldehydes and ketones?
\
Neither ketones nor aldehydes are readily oxidized.
Ketones are readily oxidized.
Both ketones and aldehydes are readily oxidized.
Aldehydes are readily oxidized.
\
Neither ketones nor aldehydes are readily oxidized.
Ketones are readily oxidized.
Both ketones and aldehydes are readily oxidized.
Aldehydes are readily oxidized.
Aldehydes are readily oxidized.
51
New cards
The word polymer means
many parts
52
New cards
Natural polymers which carry the coded genetic information that makes each individual unique are
nucleic acids
53
New cards
A copolymer is composed of
two or more different monomers
54
New cards
High-density polyethylene is composed of primarily------- chains of polyethylene in a close packing arrangement whereas low-density polyethylene is composed of primarily-------chains of polyethylene in a diffuse packing arrangement.
linear, unbranched
plystyrene
plystyrene
55
New cards
Thermoplastic polymers may be
heated and reformed
56
New cards
Which of the following types of carbon consists of one-atom-thick planar sheets of carbon?
graphene
57
New cards
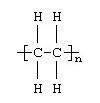
This formula in which n can be a very large number represents which of the following polymers?
polypropylene
58
New cards
Vulcanization makes rubber
hard
59
New cards
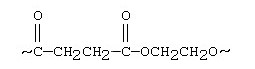
The segment of a polymer shown below represents a
polyethylene
60
New cards
Which of the following elements is NOT found in carbohydrates?
chlorine
61
New cards
Lactose could be considered to be a
milk sugar
62
New cards
From an organic chemistry perspective, carbohydrates are polyhydroxy _____________.
aldehydes and ketones
63
New cards
Complex carbohydrates are essentially polymers of
glucose
64
New cards
Animal starch is
glycogen
65
New cards
The molecular formula for glucose is
\n C6H12O6
66
New cards
The body stores most excess glucose as
fat
67
New cards
Persons with lactose intolerance ___________________
lack the enzyme for the breakdown of lactose
68
New cards
Which of the following statements about the ability of humans to digest cellulose is correct?
\
We cannot digest cellulose, because it has beta linkages between glucose units
We can digest cellulose, because it has no branches
We can digest cellulose, because it is made from glucose molecules
We cannot digest cellulose, because it is highly branched
\
We cannot digest cellulose, because it has beta linkages between glucose units
We can digest cellulose, because it has no branches
We can digest cellulose, because it is made from glucose molecules
We cannot digest cellulose, because it is highly branched
We cannot digest cellulose, because it has beta linkages between glucose units
69
New cards
A soap has a long hydrophilic tail and a charged hydrophobic head.
False
70
New cards
What ions make water "hard"?
Ca 2+
Fe 2+
Mg 2+
Fe 2+
Mg 2+
71
New cards
An agent that stabilizes the suspension of nonpolar substances in water is called a ____________.
Surfactant
72
New cards
In cleaning, soap acts as a(n) __________ between "dirt" and water.
emulsifier
73
New cards
In hard water, soaps are converted to ____________________.
insoluble salts
74
New cards
Which of the following is NOT an advantage of soap?
\
Soap is an excellent cleaner in hard water.
Soap is biodegradable.
Soap is relatively nontoxic.
Soap is derived from renewable resources.
\
Soap is an excellent cleaner in hard water.
Soap is biodegradable.
Soap is relatively nontoxic.
Soap is derived from renewable resources.
Soap is an excellent cleaner in hard water.
75
New cards
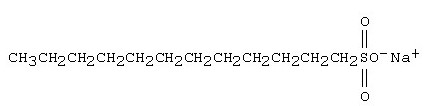
The molecule shown below is a ___________.
detergent
76
New cards
Surfactants that carry a negative charge are known as cationic surfactants.
True
77
New cards
Optical brighteners work by
absorbing ultraviolet rays and emitting blue rays
78
New cards
Bleaches work by.
changing the structure of color-producing groups called chromophores to make them colorless.
79
New cards
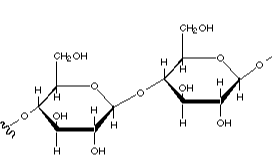
The polysaccharide shown below contains which type of linkage between monomers?
B (1-4)
80
New cards
Which statement about SPI codes (which can be found on polymer products represented by a number enclosed in a clockwise triangular arrow) is correct?
\
\
The number indicates how often the plastic has been recycled.
The number assigned to the polymer product identifies a particular group of plastics possessing a particular set of properties.
The number used indicates how difficult the item is to recycle.
The code is established by the American Chemical Society.
\
\
The number indicates how often the plastic has been recycled.
The number assigned to the polymer product identifies a particular group of plastics possessing a particular set of properties.
The number used indicates how difficult the item is to recycle.
The code is established by the American Chemical Society.
The number assigned to the polymer product identifies a particular group of plastics possessing a particular set of properties.
81
New cards
A relatively small molecule with more than 10 amino acids is called a
polupeptide
82
New cards
How many different tripeptides can be made that contain serine, glycine, and phenylalanine?
6
83
New cards
What is the chemical nature of proteins?
Proteins contain carbon, hydrogen, oxygen, nitrogen, and (usually) sulfur. About 20 different amino acids are incorporated by the genetic code. They are polyamides.
84
New cards
The interactions between different chains in a protein contribute to its __________ structure.
quaternary
85
New cards
Which of the following is NOT involved in determining the tertiary structure of a protein?
ester bonds
86
New cards
One model to explain the inhibition of enzymes involves having the inhibitor bind to the enzyme at a site distant from the active site. The enzyme then changes shape so that the substrate no longer fits into the active site
true
87
New cards
Which nucleotide is only present in RNA?
uracil
88
New cards
Which of the following amino acids can form disulfide bridges?
cystenine
89
New cards
All of the following provide adequate amounts of all essential amino acids except
corn
90
New cards
pyrimidines have only one ring, while purines have two.
true
91
New cards
Nucleotides in nucleic acids are joined by links through the
phosphate groups
92
New cards
Which food contains all of the essential amino acids? c
cheese
93
New cards
The recommended range for daily caloric intake is that a person get 20% to 35% of calories from
fat
94
New cards
It is estimated that minerals compose _________ percent of the mass of the human body.
4
95
New cards
Vitamins can be grouped into two broad categories:
fat soluble and water soluble
96
New cards
What is the safest way to supplement your vitamin A intake?
\
with an intake of Beta-carotene
with an intake of Beta-carotene
97
New cards
Fiber in the diet appears to benefit individuals with all of the following EXCEPT
scurvy
98
New cards
Which of the following is NOT a purpose for combining food with additives?
Additives have been used for all of the purposes given
99
New cards
Potassium iodide is added to table salt to prevent
goiters
100
New cards
BHA and BHT help preserve the quality of packaged food by
scavenging free radicals, consequently stopping fats from becoming rancid