WCHS Mrs. Herrick Honors Chemistry Classification of the Periodic Table
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
Who created the periodic table? How did he do it?
Dimitri Mendeleev; he arranged elements based on properties and increasing atomic mass
How did we change the periodic table from the original form?
We now organize elements by the numbers of protons
What is the periodic law?
Properties of elements change with increasing atomic number in a periodic way
What do groups (or columns) in the periodic table have in common?
Those elements have similar physical and chemical properties
What do periods (or horizontal rows) follow?
A repeating pattern
Metals
Good conductors, shiny, malleable, & easily shaped
They give away their electrons easily
Non-Metals
Poor conductors, dull, brittle, not easily shaped
They take electrons from other elements
Mettaloids (staircase)
Have properties of both metals and non-metals
They are also very good as semiconductors, used in all of our computer chips
Alakali Metals
Very reactive
Lose one electron when bonding
Alakaline Earth Metals
Reactive
Lose 2 electrons when bonding
Transition Metals
Transitions from metals to non-metals
Rare Earth Metals
After #94= man-made
Halogens
Very reactive (nonmetals)
Make bonds by gaining, or sharing electrons
Noble Gases
Inert= do not react
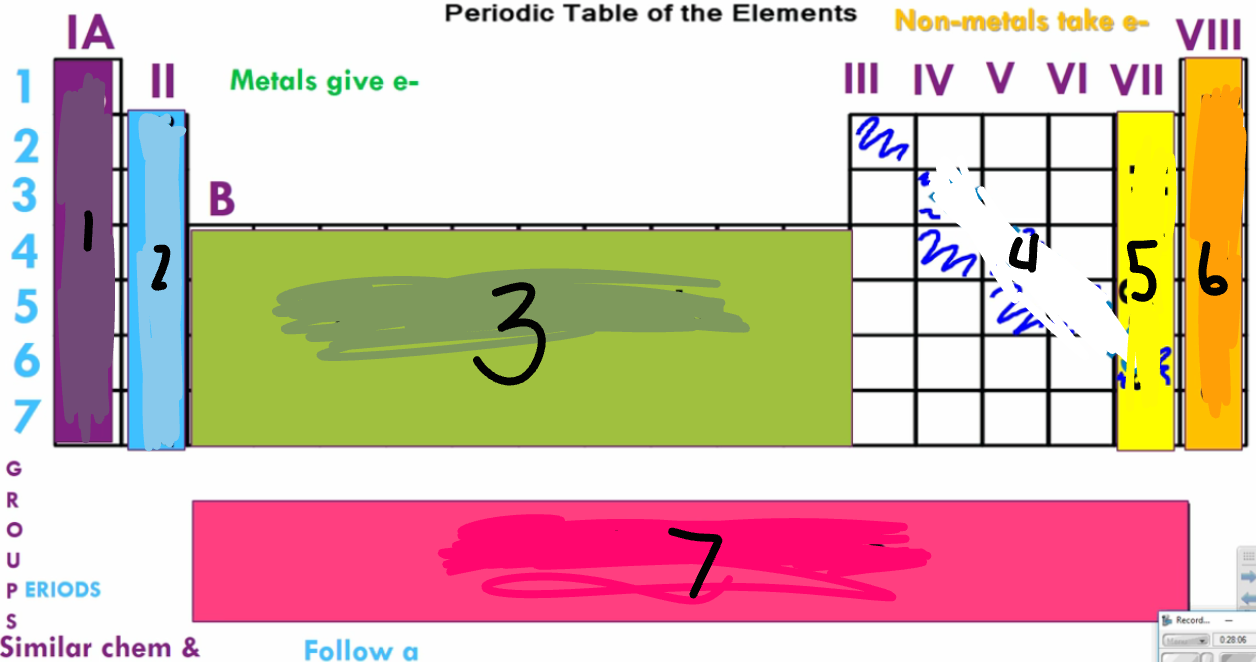
Label the image
Alakali Metals
Alkaline Earth Metals
Transition Metals
Metalloids
Halogens
Noble Gases
Rare Earth Metals
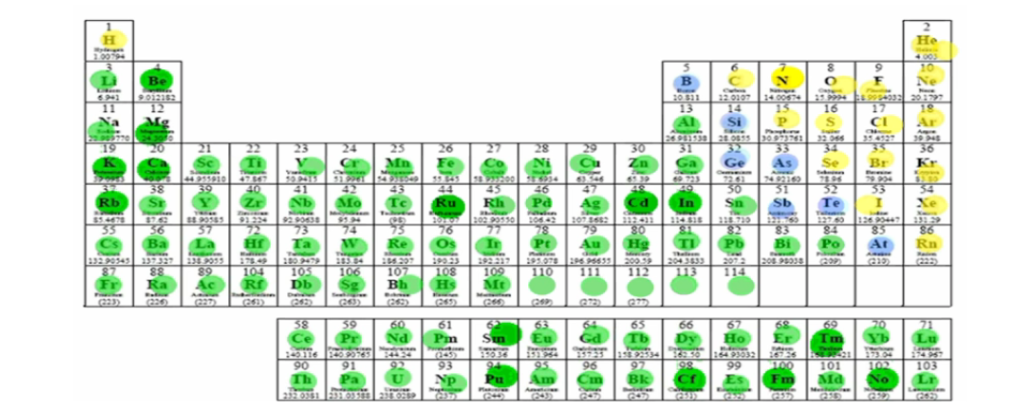
What does the yellow group represent?
Non-metals
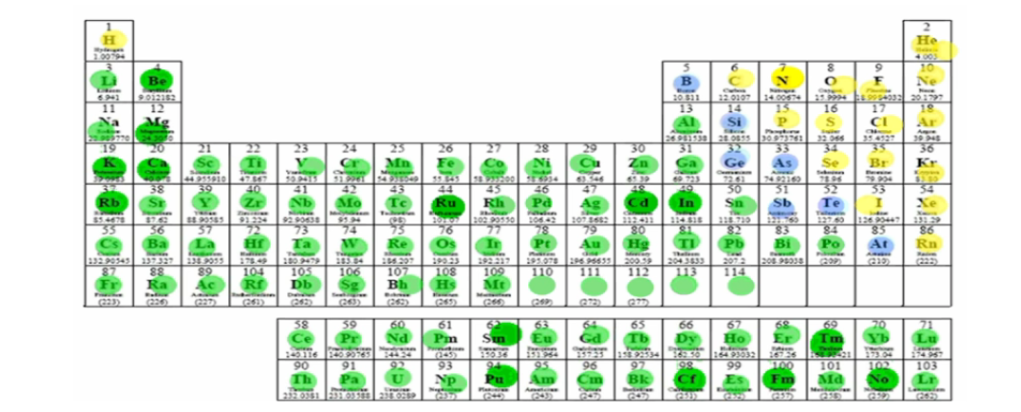
What does the blue group represent?
Metalloids
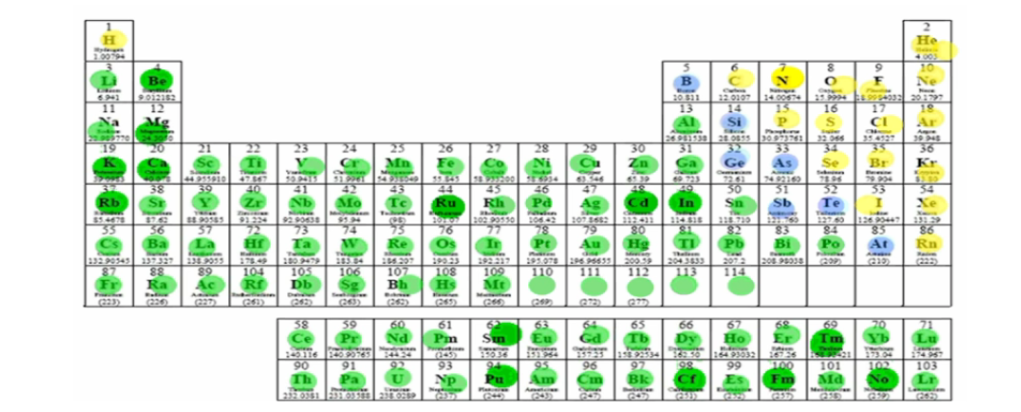
What does the green group represent?
Metals