OIA1012 ALKYNES & ALKL HALIDES
1/19
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
What is the general formula for alkynes?
The general formula is CₙH₂ₙ₋₂, indicating an unsaturated hydrocarbon with a triple bond (C≡C).
How is the C≡C bond in alkynes formed?
The bond consists of one sigma bond from the overlap of sp hybrid orbitals and two pi bonds from the overlap of unhybridized 2p orbitals.
Give an example of a pharmaceutical compound containing a C≡C bond.
Selegiline, used for Parkinson's disease and depression, contains a triple bond.
What is the IUPAC rule for naming alkynes?
Replace the suffix -ane of the corresponding alkane with -yne, and number the chain to give the triple bond the lowest possible number.
What are terminal alkynes, and why are they acidic?
Terminal alkynes have the structure R-C≡C-H. Their sp-hybridized C-H bonds have higher s-character, pulling electron density closer to the nucleus, making the hydrogen more acidic.
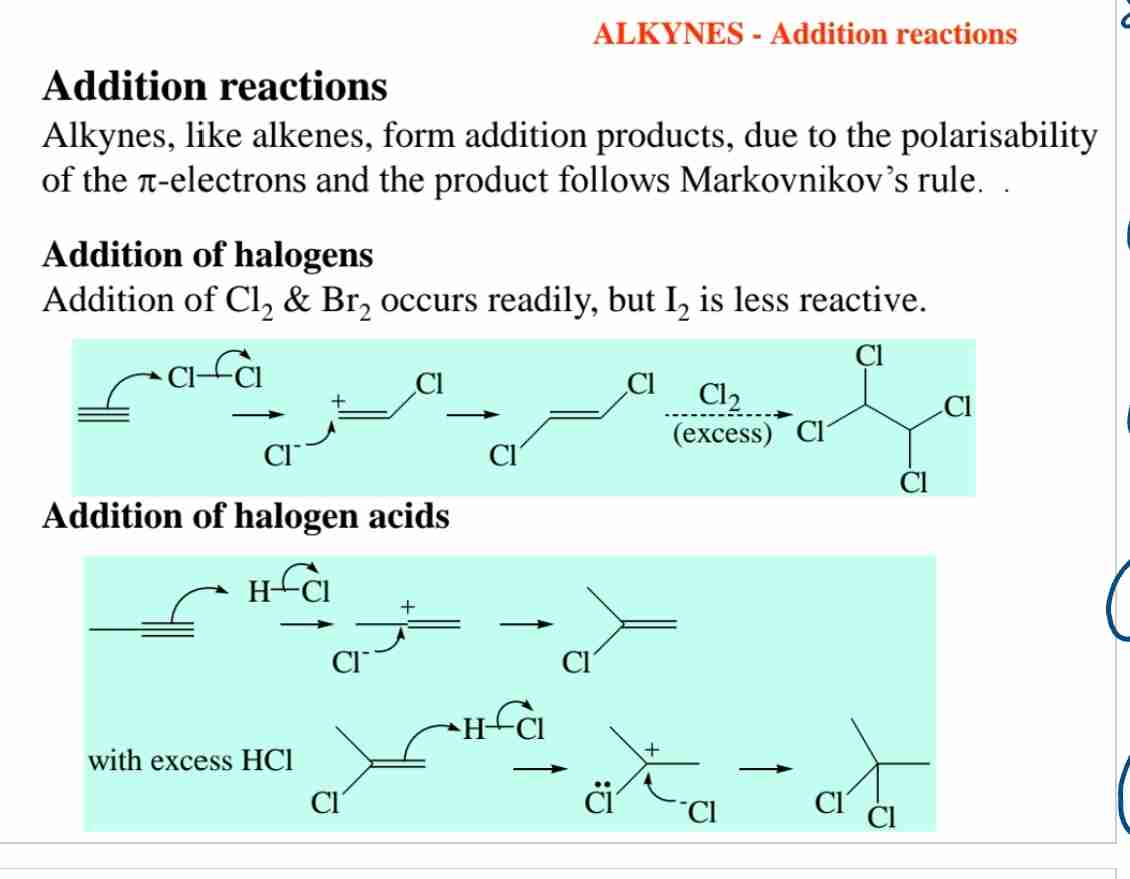
What happens in the halogenation of alkynes?
Addition of halogens like Br₂ or Cl₂ occurs, forming dihaloalkenes or tetrahaloalkanes, following Markovnikov’s rule when in excess.
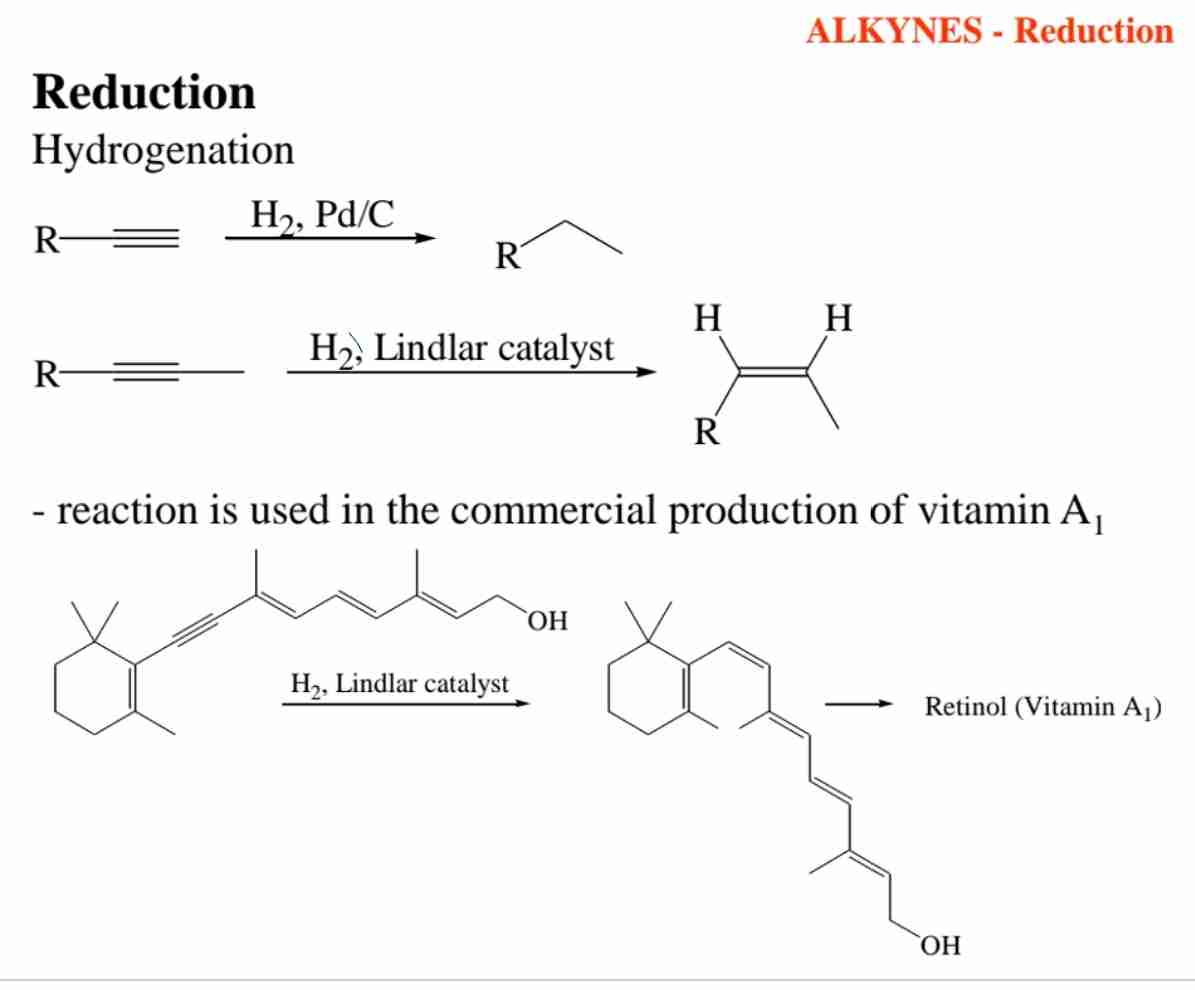
Describe the hydrogenation of alkynes with a Lindlar catalyst.
Hydrogenation with a Lindlar catalyst selectively reduces alkynes to cis-alkenes.
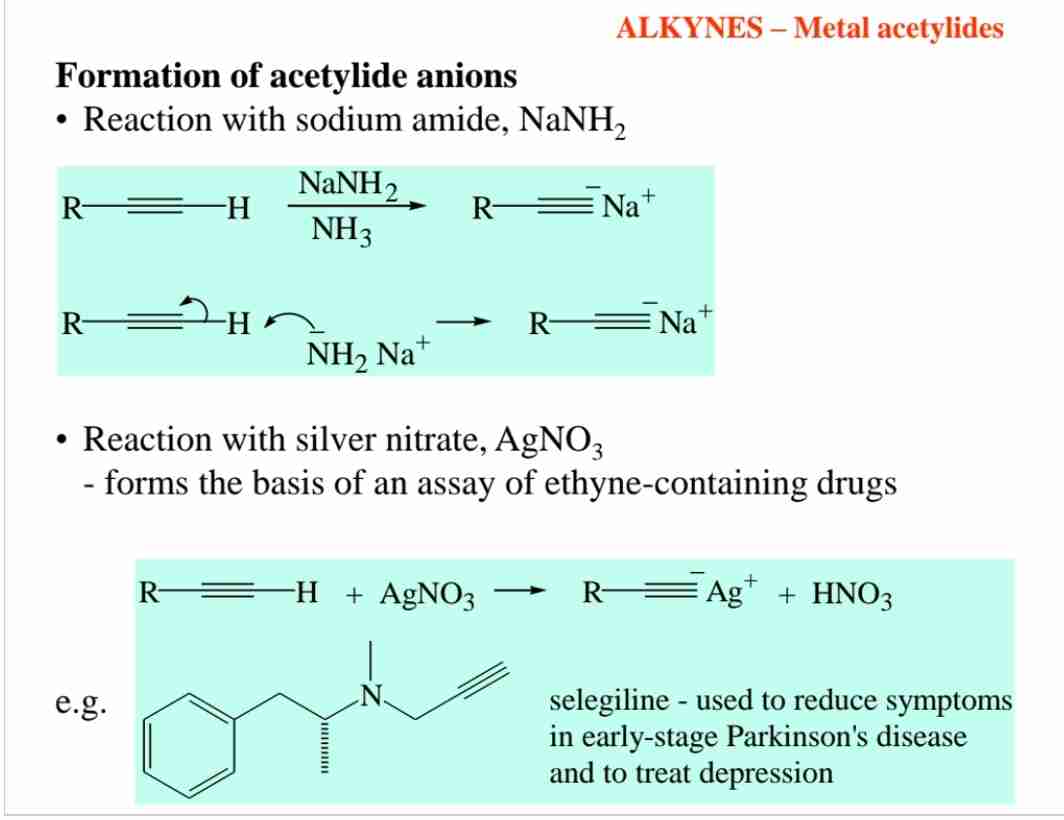
What are metal acetylides, and how are they formed?
Metal acetylides (R-C≡C⁻ M⁺) form when terminal alkynes react with strong bases like NaNH₂ or AgNO₃.
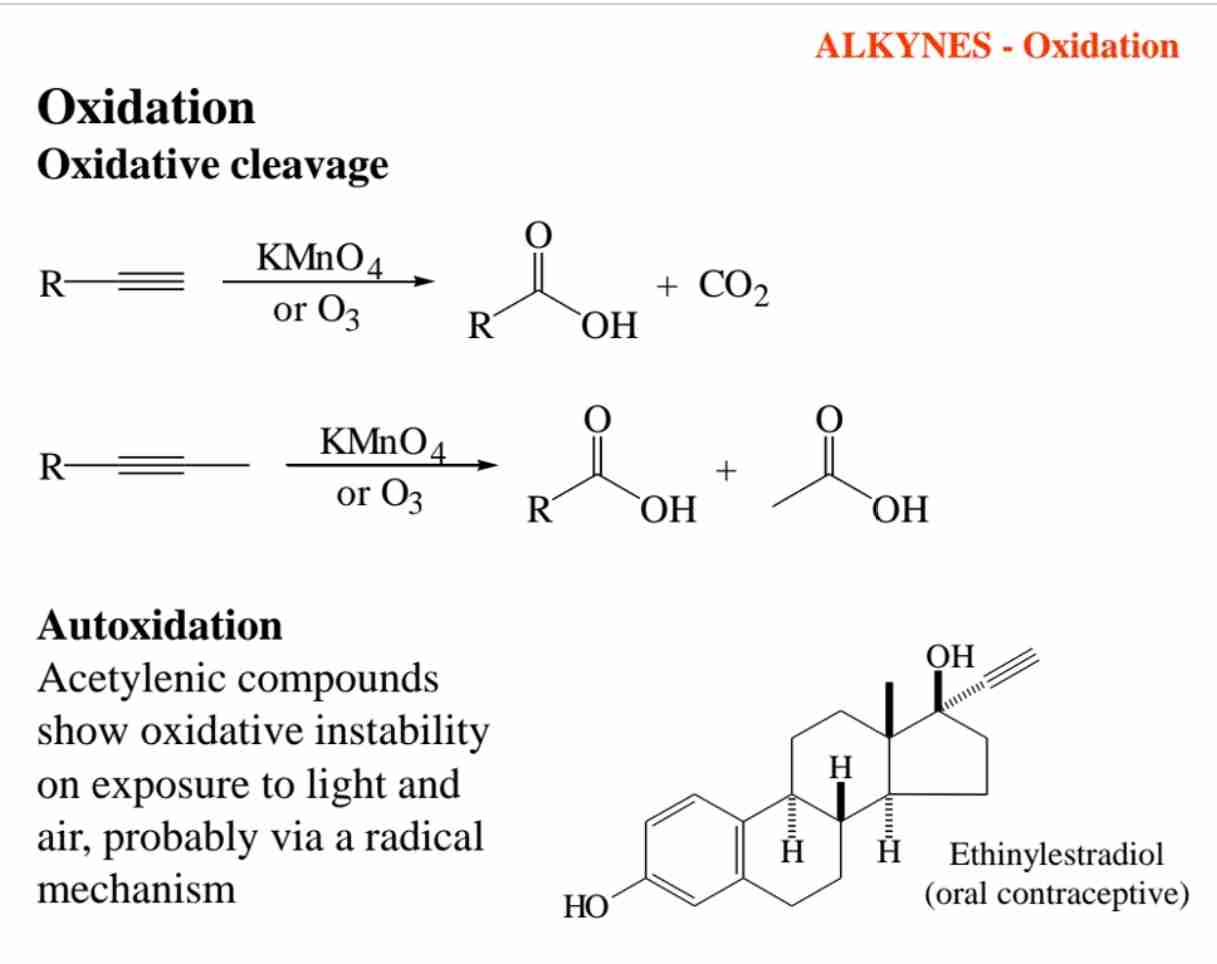
What occurs during oxidative cleavage of alkynes with KMnO₄ or O₃?
The triple bond is cleaved, producing carboxylic acids, or CO₂ if the alkyne is terminal.
Why are alkynes reactive in addition reactions?
Their polarizable pi-electrons make alkynes susceptible to electrophilic attack.
How are alkyl halides classified?
Based on the carbon atom bonded to the halogen:
Primary (1°), Secondary (2°), or Tertiary (3°).
What is the SN2 mechanism?
A bimolecular nucleophilic substitution where bond-breaking and bond-forming occur simultaneously, leading to inversion of stereochemistry.
What is the rate law for SN2 reactions?
Rate = k[RX][Nu⁻], where RX is the alkyl halide, and Nu⁻ is the nucleophile.
What factors affect the SN2 reaction?
- Less steric hindrance (1° > 2° > 3° alkyl halides).
Strong nucleophiles (e.g., HO⁻).
Polar aprotic solvents (e.g., DMSO).
What is the SN1 mechanism?
A unimolecular nucleophilic substitution involving a two-step process:
Formation of a carbocation intermediate.
Nucleophilic attack.
What is the significance of polar protic solvents in SN1 reactions?
They stabilize the carbocation intermediate, increasing the reaction rate.
Explain Zaitsev's//Saytzeff’s Rule in elimination reactions.
The major product in elimination is the more substituted alkene, as it is more stable.
What is the difference between E1 and E2 mechanisms?
E1: Unimolecular elimination with a carbocation intermediate.
E2: Bimolecular elimination with a concerted mechanism.
How do alkyl halides exhibit biological reactivity?
Their ability to alkylate DNA and proteins makes them toxic and useful in chemotherapy (e.g., nitrogen mustards).
What is the role of glutathione in detoxifying alkyl halides?
Glutathione acts as a nucleophile, substituting the halide group to form less toxic conjugates.