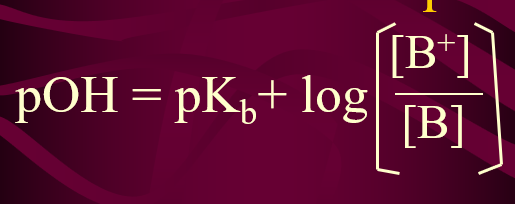L2: Preparation of Buffer Solutions
0.0(0)
Card Sorting
1/10
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
1
New cards
Buffer Solution
a solution that resists changes in pH
2
New cards
What is buffer solution made of?
* weak acid & its salt
* weak base & its salt
* weak base & its salt
3
New cards
Buffer examples
* mix acetate acid & sodium acetate
* mix ammonia & ammonium chloride
* mix ammonia & ammonium chloride
4
New cards
Acid/Base to salt ratio
1:1
5
New cards
Buffering capacity works best when
the pH is near the pKa of the acid component
6
New cards
Ka
ionization constant for weak acid
7
New cards
Kb
ionization constant for a weak base
8
New cards
Formula for pKa
pKa = -log Ka
9
New cards
Formula for pKb
pKb = -log Kb
10
New cards
Formula for pH of Buffer Solution
Henderson - Hasselbach Eq.
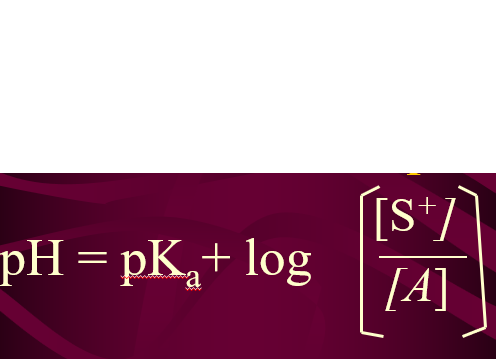
11
New cards
Formula for pOH of Buffer Solution
Henderson - Hasselbach Eq.