L2: Preparation of Buffer Solutions
BUFFER
A solution that resists changes in pH
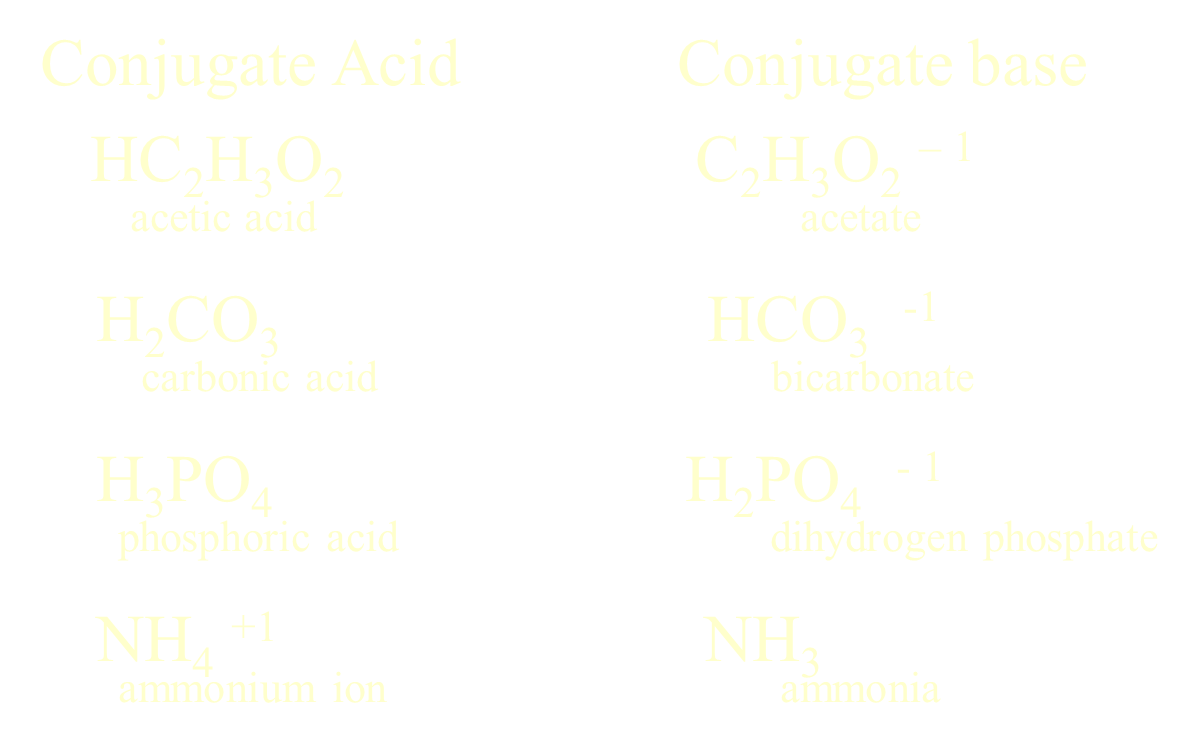
Made from the combination of
weak acid & its salt or
weak base & its salt
example
acetic acid & sodium acetate
ammonia & ammonium chloride
Buffer Solution
works best when the acid to salt ratio is 1:1
works best when the base to salt ratio is 1:1
buffering capacity of a solution works best when the pH is near the pKa of the acid component
Ka
ionization constant for a weak acid
pKa = -log Ka
ex.
Weak acid → acetic acid → Ka = 1 x 10^-5
pKa = -logKa
= -log of 1 x 10^-5
= 4.74
For the effective buffer: pH of the buffer should be closer to 4.74
pKa
pKa = -log Ka
pKb
pKb = -log Kb
Kb
ionization constant for a weak base
example: Acetic acid ionizes according to the ff. chemical equation:
Computation of Ionization Consant
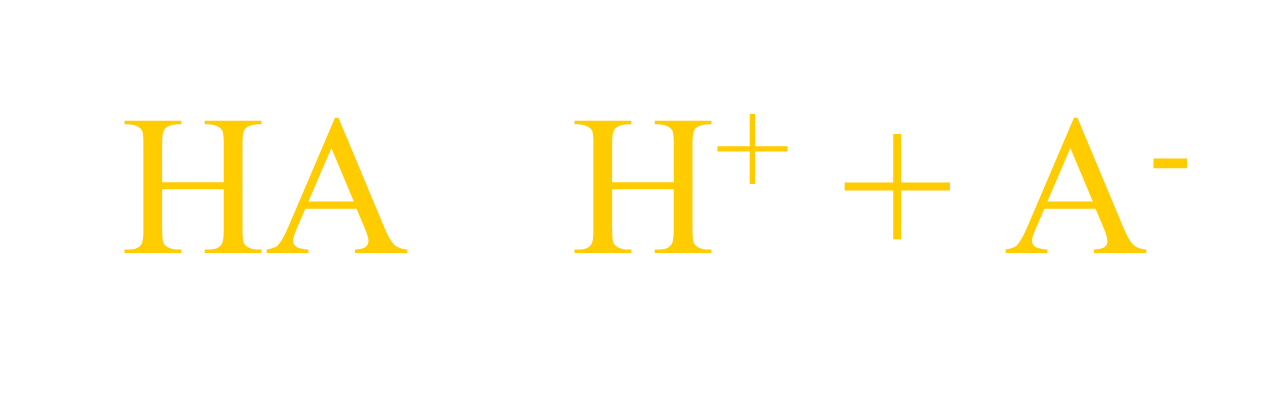
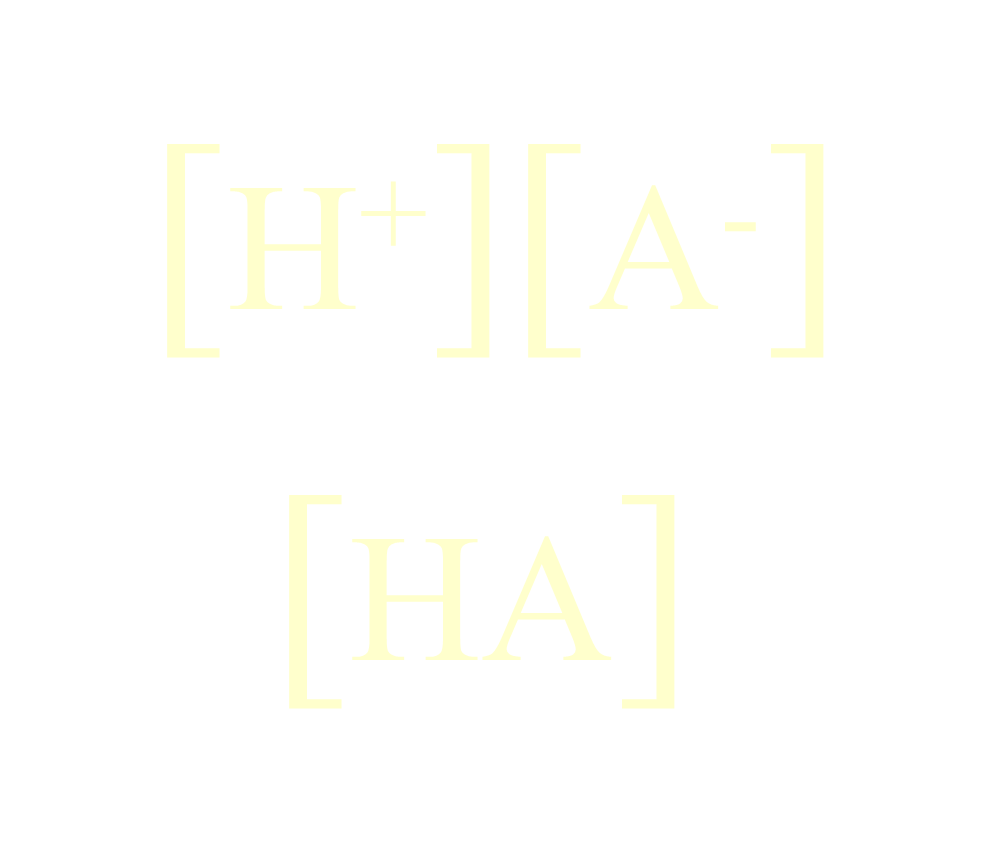
Formula to compute for the pH of Buffer Solution
Henderson-Hasselbach Eq.
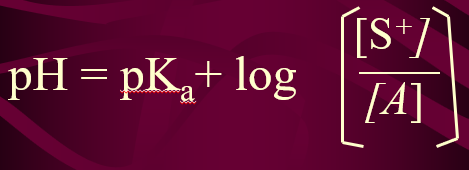
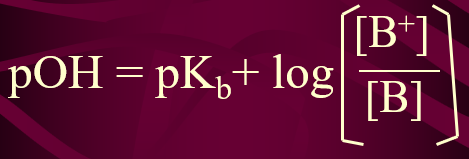
Buffering Capacity
capacity of the buffer to resist the change in the pH of a solution when an acid or alkali is added is called buffering capacity
estimated by calculating the amount of acid or alkali required to change the pH of one litre of the buffer by one unit.
Depends upon the ff. factors:
The concentration of the acid and base component of the buffer. As the concentration of acid and base components of the buffer increase, the capacity of the buffer also increases.
The pH of the Buffer can act best at pH = pKa, and its buffering range is about one pH unit above or below the pKa value