Block Exam #4
0.0(0)
Card Sorting
1/133
Earn XP
Description and Tags
Last updated 1:53 AM on 5/2/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
134 Terms
1
New cards
Electrolyte Homeostasis
1. Electrolytes
1. Sodium
2. Potassium
3. Calcium
2. HYDROGEN
2
New cards
pH Homeostasis
1. Kidney
1. Kidney can regulate what is soluble in plasma
3
New cards
Respiration
1. Respiration
2. Red blood cells (hemoglobin)
3. Lung
4
New cards
Electrolytes
1. In each compartment, total # anions = total # cations
2. Osmolality is identical in all compartments
3. Kidneys match electrolyte (Na+, K+, Cl−, bicarbonate, phosphate) excretion to ingestion
1. Input/output
4. Control of Na+ levels is important in blood pressure and blood volume
5. Control of K+ levels is important in healthy skeletal and cardiac muscle activity
6. Aldosterone plays major role in Na+ and K+ balance
5
New cards
Sodium & Volume
1. Renal regulation of salt (sodium, Na+) and water controls plasma volume
1. Volume homeostasis is dependent upon retention or excretion of water and sodium by kidneys
2. Loss/gain of Na+ = loss/gain of water
1. Regulation of Na+ is linked to volume regulation
3. No receptors directly monitor fluid or electrolyte balance
1. Blood pressure or osmolality are sensed
4. Kidneys control osmolality by
1. Excreting concentrated urine
1. Greater proportion of solutes
2. Diluting urine
1. Greater proportion of water
6
New cards
ECF Homeostasis
1. Homeostasis disturbed by ECF volume increase by fluid gain or fluid and Na+ gain
1. Increased blood volume and atrial distension
2. Increased NP release
3. Decreased aldosterone release
1. Increased urinary Na+ loss
4. Decreasd ADH release
1. Increased urinary water loss
5. Decreased thirst
1. Decreased water intake
6. Homeostasis restored
2. Homeostasis disturbed by ECF volume decreased by fluid loss or fluid and Na+ loss
1. Decreased blood volume and blood pressure
2. Increased renin secretion and angiotensin II activation
3. Increased aldosterone release
1. Increased urinary Na+ retention
4. Increasd ADH release
1. Increased thirst
1. Increased water intake
2. Decreased urinary water loss
5. Homeostasis restored
7
New cards
Salt and Water Reabsorption in Proximal Tubule
1. Reabsorption
1. Cl- transport (passive)
2. Na+ transport (active)
3. Water follows Na+ by osmosis
2. Fluid reduced to 1/3 original volume
1. Still isomotic
8
New cards
Na+ Reabsorption
1. 90% of filtered Na+ and K+ is reabsorbed early in the nephron; not regulated
1. Obligate reabsorption
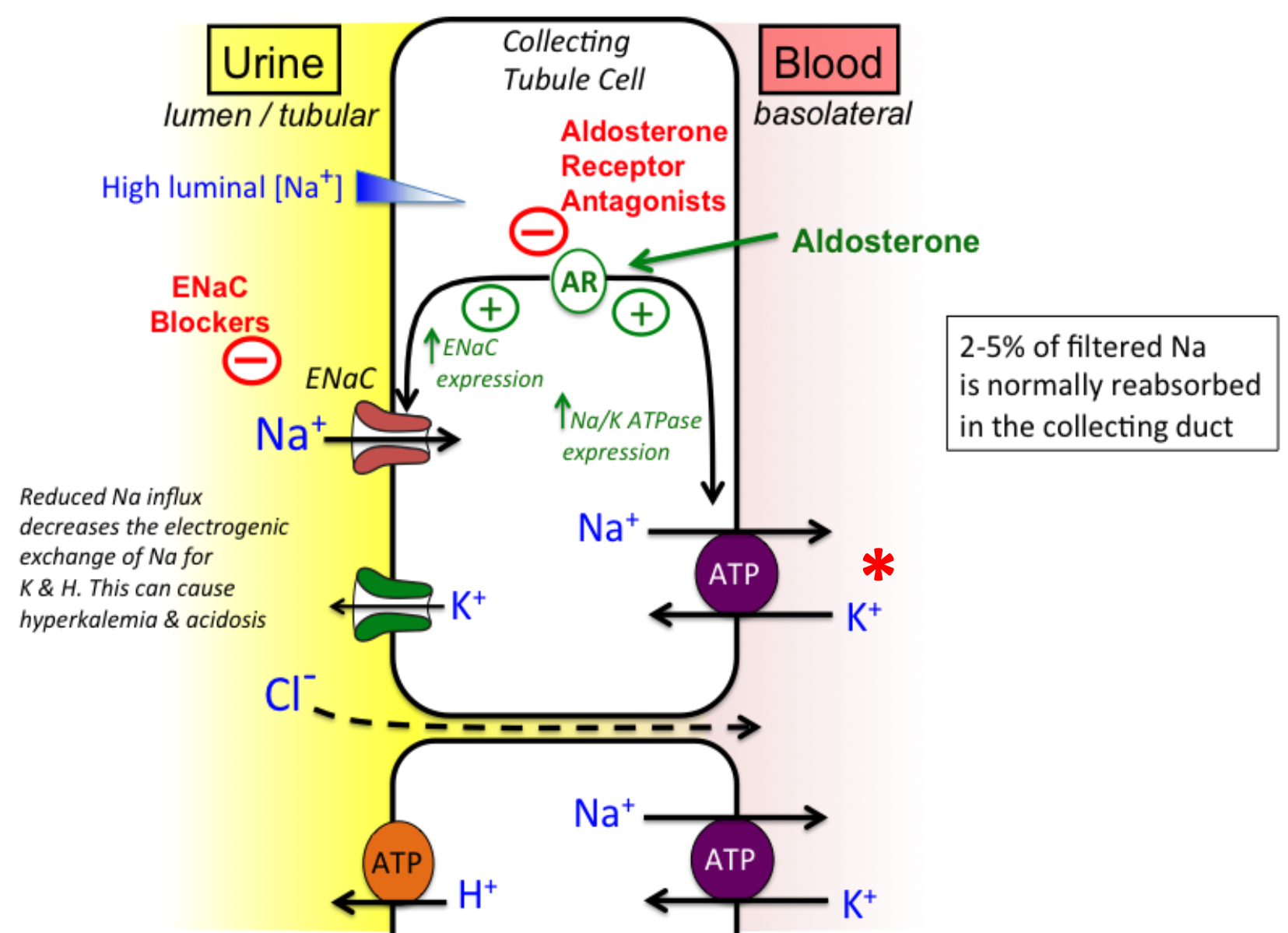
2. **Aldosterone** controls additional reabsorption of Na+ **(ENaC)** and subsequent secretion of K+ in distal tubule and collecting duct
1. **Distal tubule = Na+Cl-**
**Reabsorption of Na+ TRANSPORTERS**
1. **Na+K+ATPase** on basolateral membrane
1. **Basolateral membrane = Na+K+ATPase**
2. Drives glucose reabsorption (**SGLT**; secondary active transport)
3. Drives amino acid reabsorption (variety of secondary active transporters)
4. Conserves bicarbonate (sodium hydrogen exchanger; **NHE**; secondary active transporters)
1. Important in regulating pH
5. **Ascending limb**
1. **Na+K+2Cl-**
9
New cards
Cl- Reabsorption
1. Chloride reabsorption is coupled to sodium
1. Electroneutral
2. Working parts
1. Proximal Tubule
1. 60% reabsorbed here
2. Transporters
3. Paracellular w/ water
2. Ascending Limb
1. Na+K+2Cl-
3. Distal Tubule
1. Na+Cl-
10
New cards
Control of Sodium Excretion
1. **Distal Tubular Mechanisms**
1. Sodium reabsorbed independently of water; can alter sodium excretion when ingestion is not balanced by ingestion of water
2. **Aldosterone**
1. Increase sodium reabsorption in distal tubules and collecting duct (principal cells) via ENaC
2. Aldosterone is also regulated, in part, by AngII
1. Decrease in blood pressure increases AngII, which increases sodium reabsorption, resulting in long-term correction to sodium content and blood pressure
3. **Although aldosterone only regulates 2% of filtered sodium load, this amounts 30 g NaCl/day**
11
New cards
Regulation of Aldosterone Secretion
1. **Stimulus = decreased blood volume**
1. Increased renin secretion
2. Increased Angiotensin II production
3. Increased Aldosterone secretion
4. Mechanisms = low blood volume stimulates renal baroreceptors; granular cells release renin
2. **Stimulus = increased blood volume**
1. Decreased renin secretion
2. Decrease Angiotensin II production
3. Decreased Aldosterone secretion
4. Mechanisms = increased blood volume inhibits baroreceptors; increased Na+ in distal tubule acts via macula densa to inhibit release of renin from granular cells
3. **Stimulus = increase in K+**
1. No renin secretion
2. Angiotensin II production doesn’t change
3. Increased Aldosterone secretion
4. Mechanisms = direct stimulation of adrenal cortex
4. **Stimulus = increase in sympathetic nerve activity**
1. Increased renin secretion
2. Increased Angiotensin II production
3. Increased Aldosterone secretion
4. Mechanisms = a-adrenergic effect stimulates constriction of afferent arterioles; B-adrenergic effect stimulates renin secretion directly
12
New cards
Aldosterone-ENaC
1. Redcued Na+ influx decreases the electrogenic exchange of Na+ for K+ and H+
1. Can cause
1. Hyperkalemia
2. Acidosis
13
New cards
Juxtaglomerular Apparatus-Aldosterone
1. Located where the afferent arteriole contacts the distal tubule
2. A decrease in plasma Na+ results in a fall in blood volume
1. Sensed by juxtaglomerular apparatus
2. Granular cells secrete renin into afferent arteriole
3. Converts angiotensinogen into angiotensin I
4. Angiotensin-converting enzyme (ACE) converts this into angiotensin II
3. Angiotensin II stimulates the adrenal cortex to secrete Aldosterone
1. Promotes reabsorption of Na+ from cortical collecting duct
2. Promotes secretion of K+
3. Increases blood volume and increases blood pressure
14
New cards
Na+, K+, and H+
1. Increases in sodium reabsorption drive potassium secretion (and excretion)
1. Membrane potential difference created by Na+ reabsorption driving K+ through K+ channels
2. Stimulation of renin-angiotensin-aldosterone system by water and Na+ in filtrate
3. Increased flow rates activates K+ channels
2. **Acidosis** stimulates the secretion of H+ and inhibits secretion of K+ ions
1. Acidosis can lead to hyperkalemia
3. **Alkalosis** stimulates the secretion and excretion of K+
1. Alkalosis can lead to hypokalemia
4. **Hyperkalemia** stimulates secretion of K+ and inhibits secretion of H+
1. Hyperkalmia can lead to acidosis
15
New cards
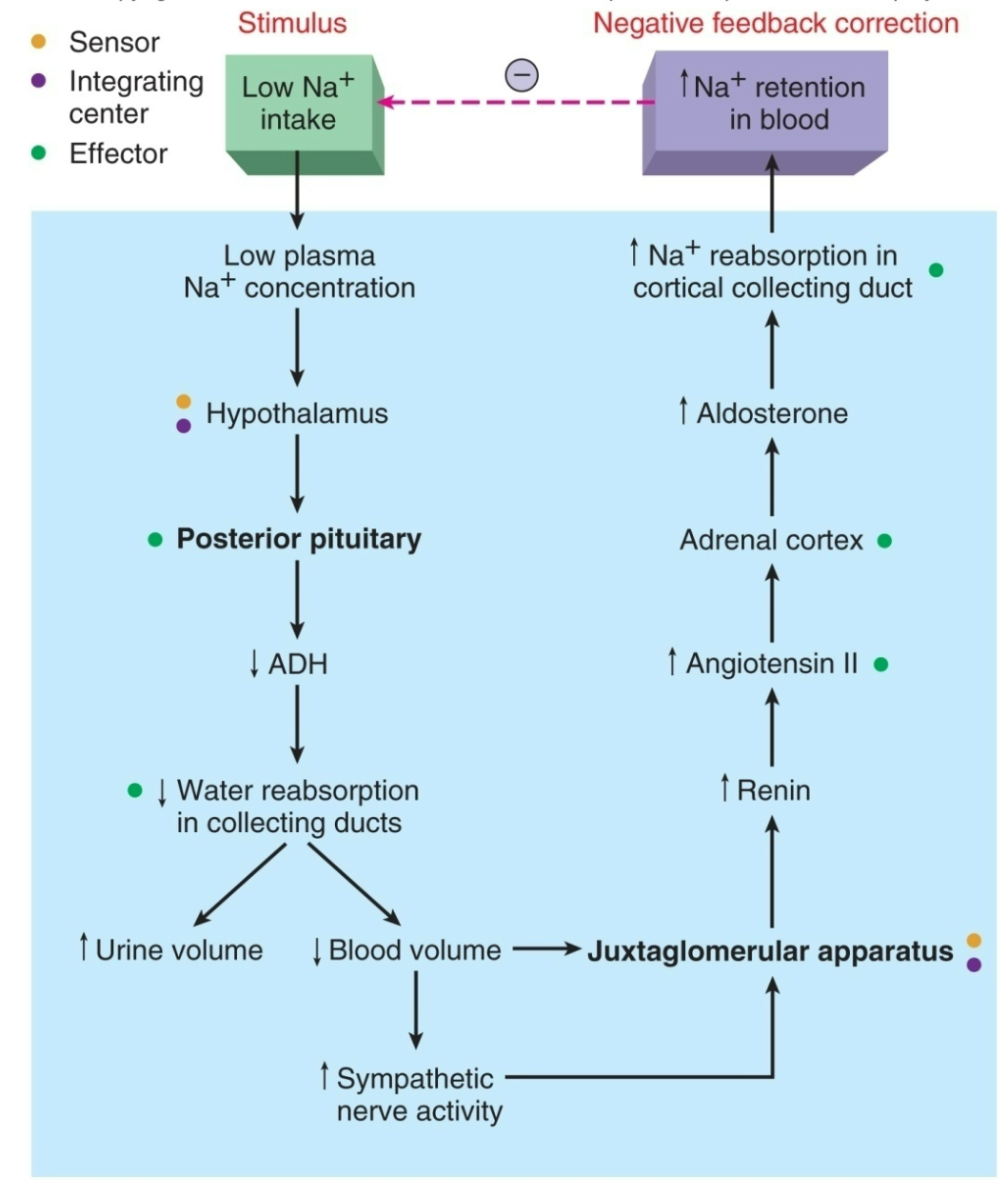
Homeostasis of Plasma Na+
1. Stimulus = low Na+ intake
2. Causes low plasma Na+ concentraion
3. Hypothalamus senses
4. Posterior pituitary gland lowers ADH levels
5. Water reabsorption in collecting ducts lowers
1. Increase in urine volume
2. Decrease in blood volume
1. Increase in sympathetic nerve activity
2. Signals Juxtaglomerular Apparatus
6. Juxtaglomerular Apparatus
1. Increases renin secretion
7. Increases Angiotensis II
8. Adrenal cortex
9. Increase Aldosterone secretion
10. Increases Na+ reabsorption in cortical collecting duct
11. Increases Na+ retention in blood
1. Start over from beginning
16
New cards
Electrolyte II: Potassium
1. Regulation of K+ adds an additional variable
1. Most K+ is intracellular
1. Cellular shifts (in to out; out to in) must be considered
17
New cards
Potassium
1. Input
1. Dietary potassium
1. Major source
2. Output
1. Not normally significant, but can become so in abnormal states
1. Diarrhea and vomiting
2. Excretion via kidneys
1. Most important quantitatively
2. Under physiological control
18
New cards
Regulation of Potassium
1. Most K+ is in intracellular fluid (ICF; IC)
1. Small fraction (2%) in extracellular fluid (ECF) is evenly distributed in plasma and interstitial fluid
2. Changes in \[K+\]plasma reflect
1. Changes in \[K+\]body
2. Shifts of K+ into or out of ICF (skeletal muscle)
1. Dependent upon insulin, epinephrine, or reciprocal movement of H+
1. Insulin promotes intracellular accumulation of K+ (fed state)
2. Epinephrine stimulates cellular uptake via Na+K+ATPase (increased extracellular flux from action potentials in muscle cells)
3. Acidemia (increased H+) stimulates potassium efflux
3. **Small changes in [K+]plasma can have profound effects on cellular function, especially the heart, control of [K+]plasma is critical**
19
New cards
Importance of Regulating \[K+\]ECF: Resting Membrane Potential
1. **Hypokalemia**
1. Low \[K+\]ECF
2. Negative membrane potential
3. Low excitability
4. Hyperpolarization
1. Inside more negative than outside
2. **Hyperkalemia**
1. High \[K+\]ECF
2. Positive membrane potential
3. High excitiability
4. Depolarization
1. Inside more positive than outside
20
New cards
K+: Kidney
1. \[K+\]plasma makes up < 10% of \[Na+\]plasma
1. Amount filtered is proportionately lower
2. K+ ingested = K+ excreted
3. Usually >90% filtered K+ is reabsorbed
1. About 70% in proximal tubule and 20% in ascending limb of Henle’s loop
4. Conditions can occur where K+ excretion > K+ filtered
1. Implies tubule is capable of **K+ secretion**
2. **Amount excreted is controlled by how much is secreted**
21
New cards
K+: Proximal Tubule
1. Reabsorption relatively fixed
1. Does not vary much with changing physiological events
2. Precise mechanisms not clear, but may include
1. Early proximal tubule
1. Lumen is relatively electronegative
2. K+ brought in by ATP-dependent pump (active transport)
2. Late proximal tubule
1. Lumen slightly electropositive
2. Favors passive movement of K+ via **paracellular route**
3. **This is thought to be primary** \n **mechanism.**
3. “Leaky” tight junctions and influx of Na+ and water may carry K+
22
New cards
K+: Loop of Henle (Thick Ascending)
1. K+ reabsorbed via transcellular route
1. Crosses luminal membrane via **Na+K+2Cl-cotransporter**
2. Then crosses basolateral membrane down concentration gradient
2. **Proximal tubule and loop of Henle mechanisms account for reabsorption of all filtered K+ (90%)**
23
New cards
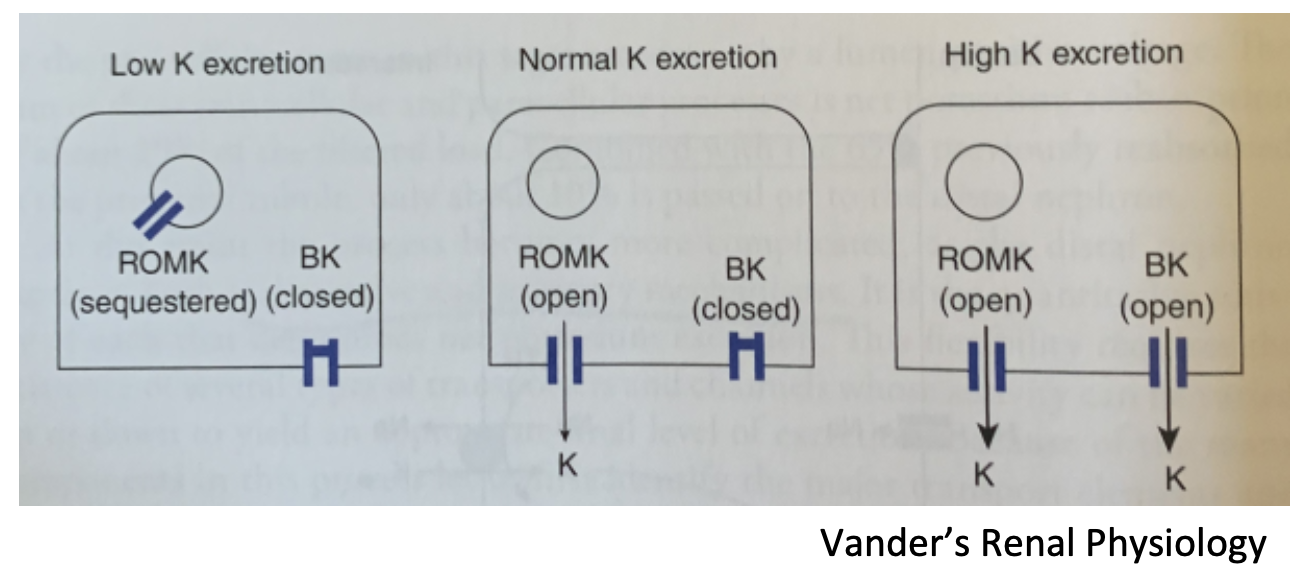
Potassium Channels
1. **ROMK**
1. Renal Outer Medullary Potassium channel
2. Secrete K+; in principal cells
3. Rate of secretion is dependent upon uptake of Na+ via ENaC (aldosterone) in effect
1. A functional exchange of reabsorbed Na+ for secreted K+
2. **BK**
1. Big capacity (maxi-K)
2. Calcium, magnesium, voltage-dependent
3. In type A intercalated cells, these cells can secrete K+
3. **Vander’s Renal Physiology**
24
New cards
K+: Distal Tubule & Collecting Duct
1. **Principal cells (collecting duct)**
1. Na+-K+ ATPase in basolateral membrane maintains low \[Na+\] intracellular and high \[K+\] intracellular \n concentration gradient for K+ diffusion into tubular lumen
2. Reabsorption of Na+ from lumen to the interstitium creates a negative lumen electrical gradient
1. Favors K+ secretion into lumen (ROMK)
3. **Under control by aldosterone**
1. **Increase in [K+]plasma directly stimulates** \n **release of aldosterone from adrenal cortex**
2. **Stimulates Na+ reabsorption**
3. **Stimulates K+ secretion**
25
New cards
K+: Medullary collecting duct
1. **Intercalated cells (type A; collecting duct)**
1. Major role is active pumping of H+ into lumen
1. Acidifying urine
2. Reabsorbing new HCO3-
2. **Can secrete K+ through BK channels**
1. **May be limited by H+ secretion**
26
New cards
Potassium Secretion
1. **Aldosterone-independent**
1. Increase in plasma K+ triggers an increase in \n number of potassium channels in cortical collecting duct
2. When blood K+ levels drop, these channels are removed from membrane
2. **Aldosterone-dependent**
1. Increase in plasma K+ triggers adrenal cortex to \n release aldosterone
2. This increases K+ secretion in distal tubule and collecting duct
27
New cards
Renal Regulation of Na+, K+, H+
1. General rule
1. For whole organism, renal handling of Na+ has priority
1. Regulation of Na+ balance will often occur at \n expense of H+ or K+
2. Provided Na+ balance is satisfied, pH regulation \n often will occur at the expense of \[K+\]ECF regulation
3. Possible reason for “low priority” for regulation of\[K+\]ECF
1. K+ is mainly in ICF
2. Cells regulate the bulk of body potassium
28
New cards
Hyperkalemia
1. **Increased plasma K+**
1. In presence of continued intake K+ retention \n may increase if (\* = medications)
1. Renal disease with impaired K+ secretion
2. Adrenal dysfunction leads to deficiency \n of aldosterone
3. \*Angiotensin converting enzyme (ACE) inhibitors block AngII-mediated release of aldosterone by blocking AngI conversion into AngII
4. \*Direct block of aldosterone’s effect on distal tubule
5. \*Inhibitors of distal tubule Na+ transport
2. **ICF to ECF shift**
1. Severe systemic acidosis
1. K+ leaves cells by exchange with H+ which binds to intracellular buffers
2. Insulin deficiency
3. Release of K+ from damaged cells (Rhabdomyolysis)
3. May be multiple factors underlying hyperkalemia
1. Renal dysfunction always confers higher risk
29
New cards
Hypokalemia
1. **Decreased plasma K+**
1. From intestine due to vomiting, diarrhea, etc.
2. From kidney due to renal disease, diuretic treatment or increased aldosterone production
2. **ECF to ICF shift**
1. Primary alkalotic state
2. Correction of high blood sugar indiabetes by insulin treatment
30
New cards
KEY IDEAS
1. Potassium reabsorption is nearly complete by end of loop of Henle, is a fixed property, and is not independently regulated
2. **Distal system is only part of nephron with regulated handling of K+ responding to body’s need to lose or gain K+**
3. K+ secretion by **Principal cells** depends upon gradients produced by active Na+ reabsorption and is thus secondary to Na+
4. H+ pumped out by **Intercalated cells** will diminish potential gradients, thus limiting capacity to excrete K+
31
New cards
Electrolyte III: Calcium/Phosphate
1. Calcium
1. Relatively low extracellular and intracellular (in \n organelles-SR)
2. Stored in bone
32
New cards
Calcium (Ca2+) Functions
1. Neuronal excitability
2. Muscle contraction
3. Mineralization of bones and teeth
4. Signaling
5. Intercellular adhesion
6. Blood coagulation
33
New cards
Regulation of Calcium and Phosphate
1. **Almost all of the body’s calcium is in bone**
1. Accompanied by phosphate
1. **Hydroxyapatite**
2. Regulation of these is coordinated
2. Although intracellular calcium is critical for several processes, it is tightly regulated
1. High levels of intracellular calcium are toxic
3. Phosphate is an important signaling mediator
1. Remember activation of MLCK in smooth muscle)
34
New cards
Calcium: Input and Outputs
1. BONE 990,000 mg
2. Daily calcium balance
1. Intake = Urinary + Fecal loss (output)
3. Free Ca2+ is freely filtered
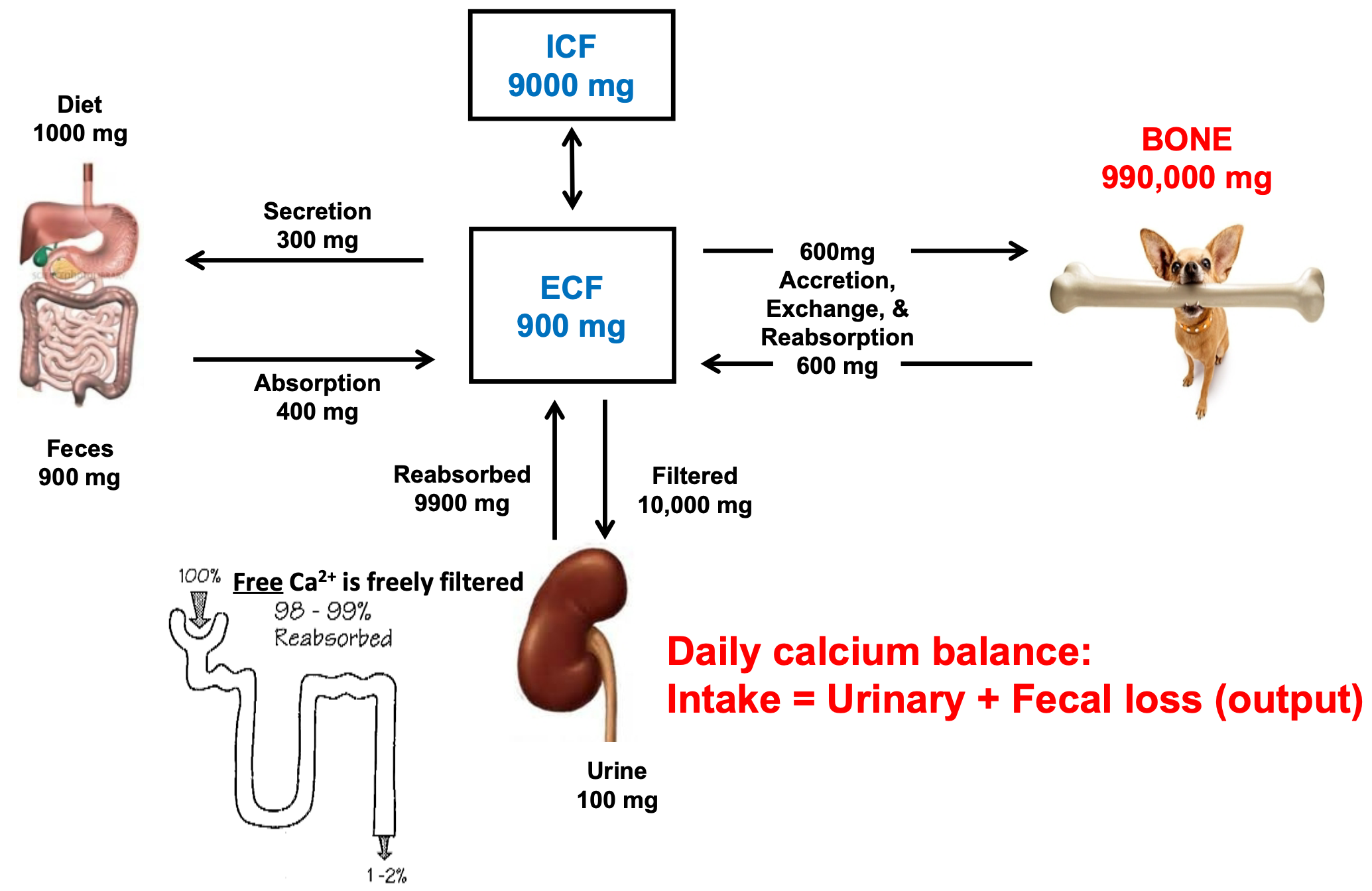
35
New cards
Calcium: ECF
1. Free, ionized Ca2+
1. Physiologically Important
2. Bound to plasma proteins (e.g. albumin)
1. In interstitial fluid, albumin is low, so all Ca2+ is free
3. Normal pH = \[Ca2=\]
1. \[Ca2+\] = \[Ca-Pr\]
4. **Acidosis**
1. Lower pH
2. Fewer (-) binding sites since more are taken up \n by binding H+ = increase in \[Ca2+\] levels
5. **Alkalosis**
1. Higher pH
2. More (-) binding sites available = decrease in \[Ca2+\]
6. **Ca2+ entry** via
1. Gut absorption
2. Release from bone
7. **Ca2+ loss** via
1. Entering bone
2. Urinary excretion
8. The small proportion of total body calcium that is in the ECF is regulated within a narrow range
36
New cards
ECF Phosphate
1. Two major forms
1. Di-H
1. H2PO4-
2. Mono-H-
3. HPO4(2-)
2. Does not exist at pH 7.4
1. PO4(3-)
3. At pH 7.4
1. CHECK IMAGE
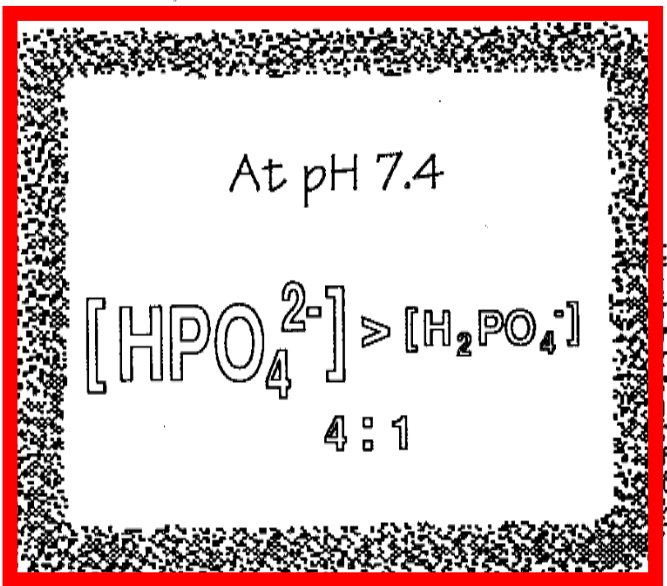
37
New cards
Calcium and Phosphate
1. Whether calcium and phosphate are deposited in bone (precipitate from solution) or are resorbed from bone (go into solution) depends on product of their concentrations rather than on their individual concentrations
2. When the product exceeds a certain number (solubility product) precipitation
1. This is OK in bone, but NOT in ECF
3. Under normal conditions the ECF product of calcium times phosphate is close to the solubility product
1. An increase in interstitial fluid concentration of either Ca2+ or phosphate increases bone mineralization
2. A malignant increase in concentration of calcium or phosphate due to chronic renal disease or rhabdomyolysis can cause the precipitation of calcium phosphate within tissues
38
New cards
Parathyroid Hormone (PTH)
1. Release of Ca2+ from bone
2. Increased renal tubular loss of PO4-
3. Increased renal tubular reabsorptionof Ca2+
4. Stimulation of production of bioactive form of Vitamin D
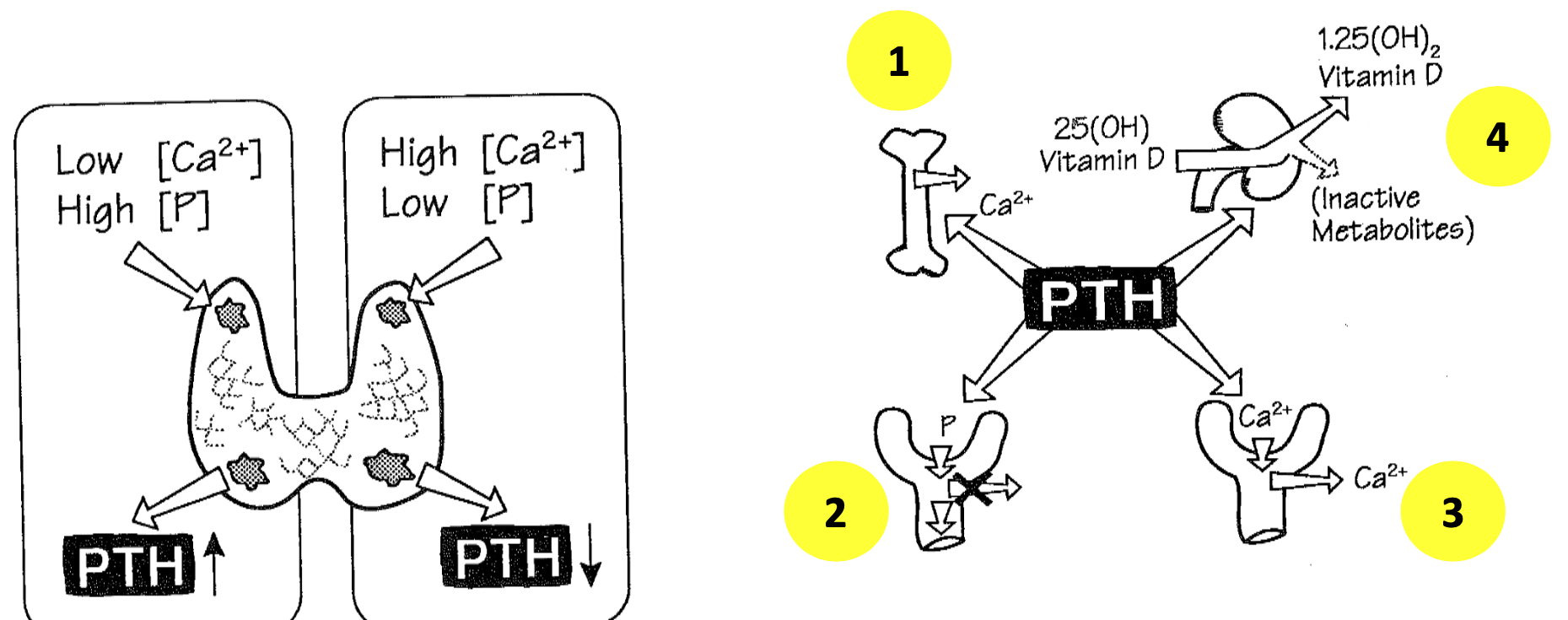
39
New cards
Calcium and Phosphate: Kidney
1. 98-99% of Ca2+ reabsorped
2. 85% of Phosphate reabsorped
3. PTH increases Ca2+ reabsorption (TRPV channels) which decreases phosphate reabsorption
4. As phosphate passes through nephron, it traps H+ being pumped into lumen from tubular cells
40
New cards
Vitamin D
1. Diet and skin
2. Chloecaliciferol
3. Liver
1. 25 OH Chloecaliciferol
4. Kidney
1. Final step is stimulated by low plasma calcium
2. PTH
3. Active form = 1.25 (OH)2 Chloecaliciferol
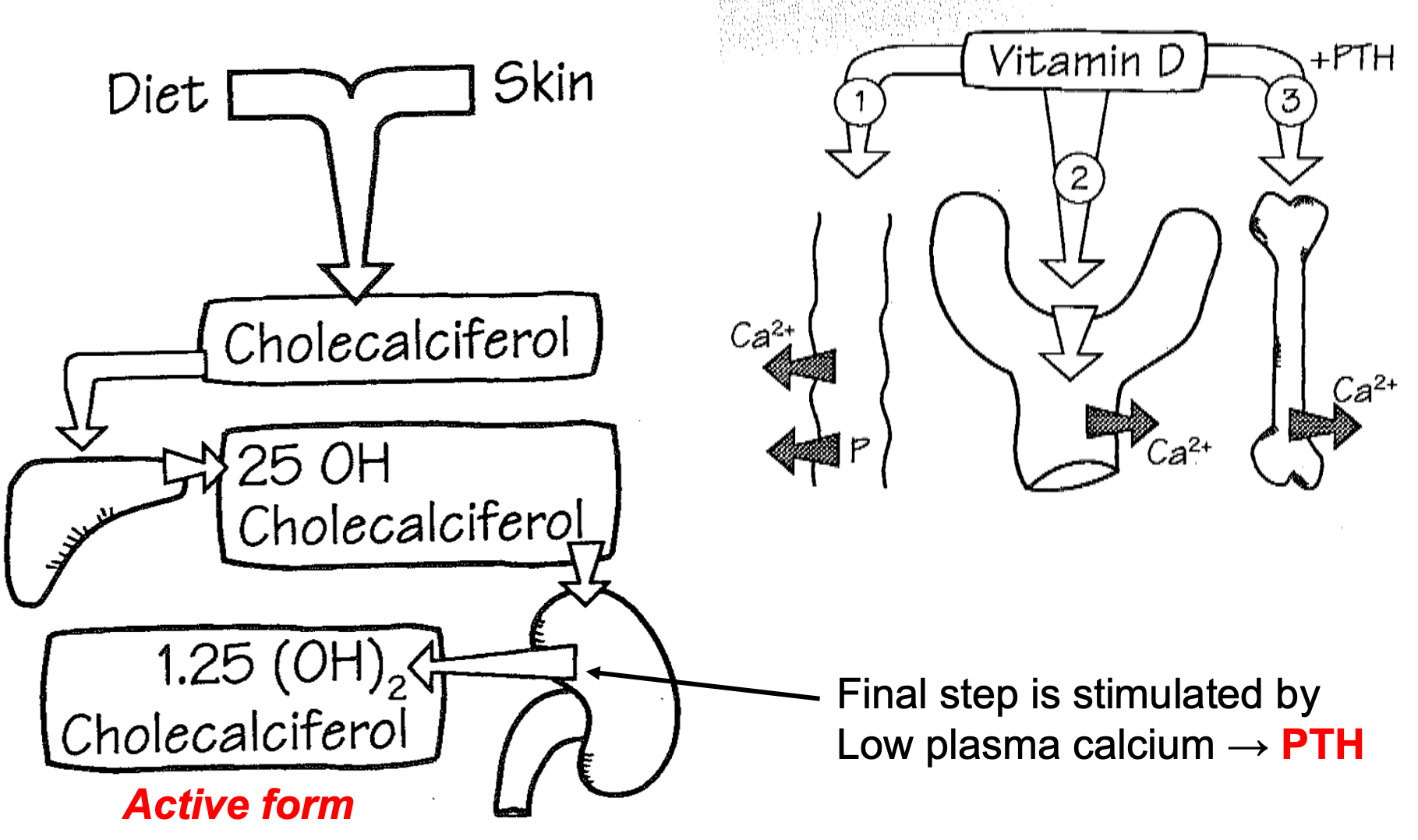
41
New cards
Calcium: Absorption
1. Calcitriol (form of Vitamin D)
1. Promotes Ca2+ absorption from the intestine by \n stimulating synthesis of Ca2+ binding protein
2. Lumen, Intestinal enterocyte, blood
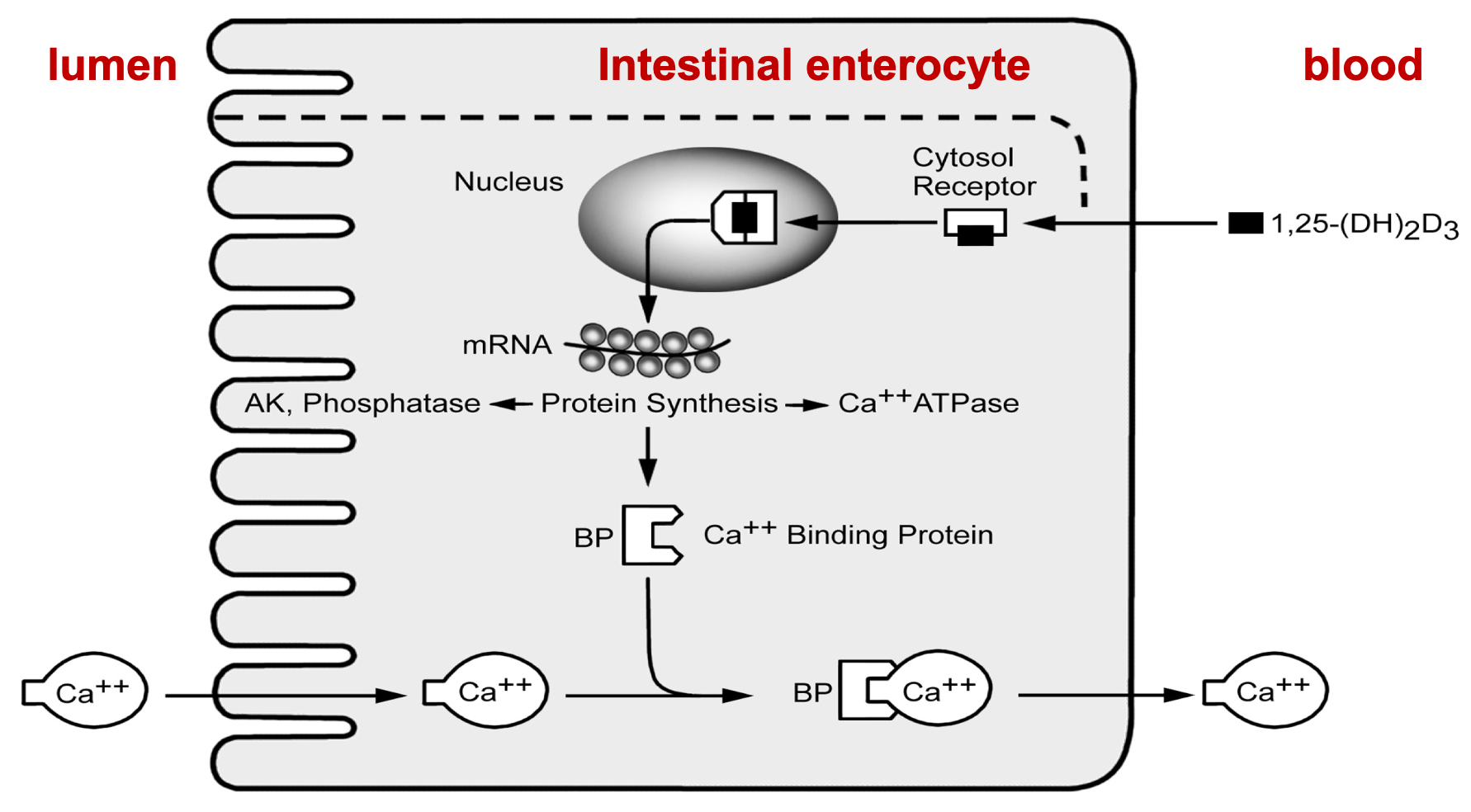
42
New cards
Plasma Ca2+: Regulation
1. Increased plasma Ca2+
1. Lower PTH
2. Increase in Vitamin D
2. Decreased plasma Ca2+
1. Thyroid secretes Calcitonin
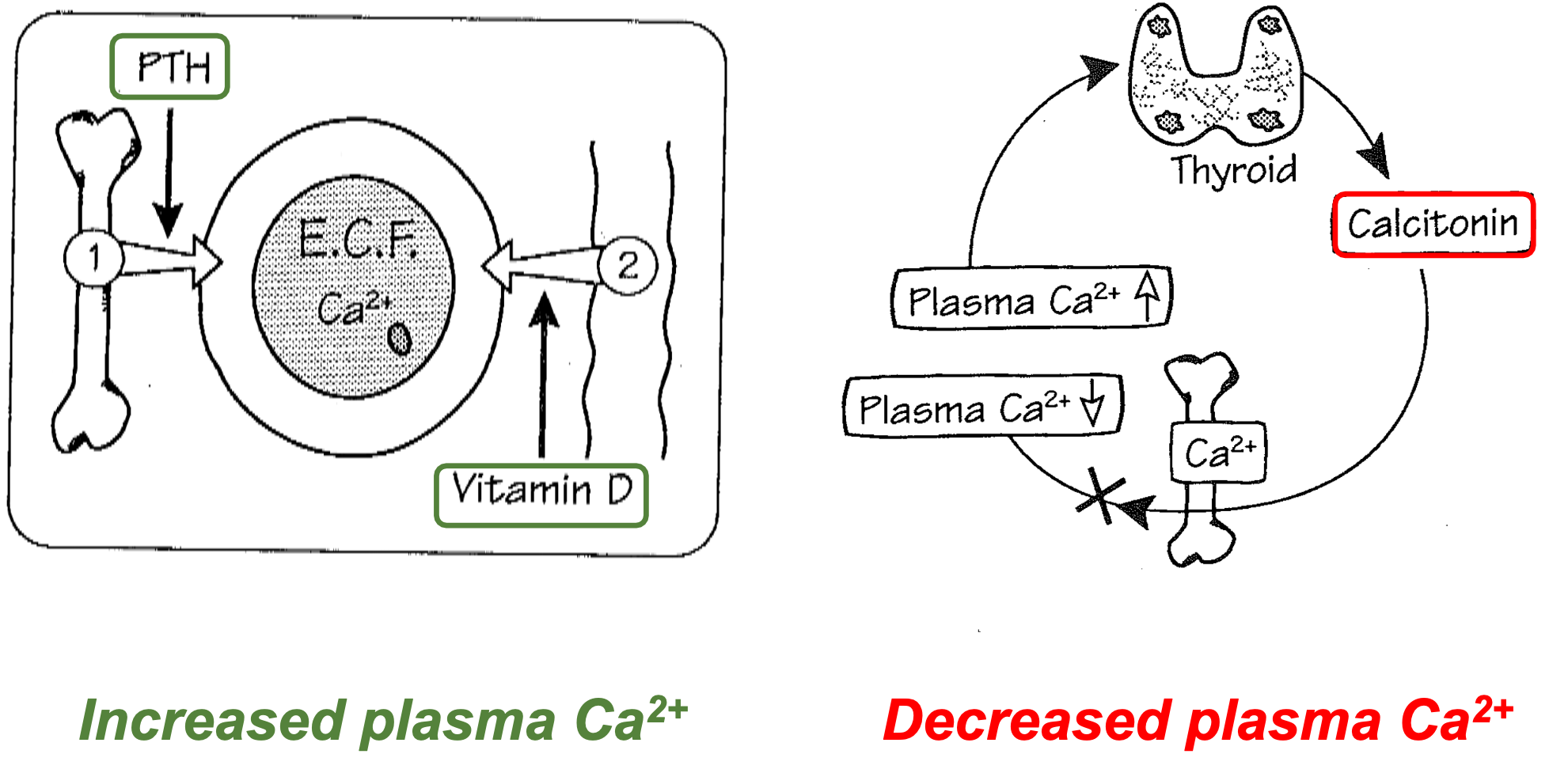
43
New cards
Hypocalcemia
1. Vitamin D deficiency and Malabsorption
2. PTH deficiency
3. Alkalosis
4. Failure of renal phosphate excretion
5. Failure of conversion of Vitamin D
6. Causes
1. Tetany
2. Convulsion
44
New cards
Hypercalcemia
1. Increase intestinal absorption of Vitamin D
1. Excess or increased sensitivity of Vitamin D
2. Increase release from bone
1. PTH-secreting tumor
1. PTH
2. Bone disease (cancer)
3. PTH-like substances from extra-parathyroid tumors
3. Causes
1. Nasuea
2. Peptic ulcers
3. Mental disturbances
1. Depression
4. Renal failure
5. Renal stones
6. Polyuria
7. Constipation
8. Soft tissue calcification
45
New cards
pH I: Principles of Acid-Base Balance
1. Delineate the systems important in regulating pH
1. Intracellular buffers
2. Extracellular buffers (CO2-bicarbonate)
46
New cards
Acid–Base Balance
1. Normal pH of plasma is 7.38–7.42
1. pH = log 1/\[H+\]
2. Inverse relationship
1. As \[H+\] increases, pH decreases
2. H+ concentration is tightly regulated
3. Abnormal pH affects
1. Cardiac activity
2. Membrane permeability
3. Action potential – myelinated neurons
4. Oxygen-Hemoglobin dissociation curve
5. Enzyme activity
4. pH disturbances
1. Associated with K+ disturbances
47
New cards
Background (Acid-base balance processes)
1. Acid-base balance involves two related processes
1. Matching the excretion of acid/base equivalents to \n their input – balance
2. Regulating the ratio of weak acids to their conjugate bases in buffer systems - pH
2. **pH BALANCE IN THE BODY**
1. **Fatty and Amino acids = H+ INPUT**
1. Diet
2. Plasma pH 7.38–7.42
3. Buffers
1. HCO3– in extracellular fluid
2. Proteins, hemoglobin, phosphates in cells
3. Phosphates, ammonia in urine
4. Ventilation
5. **CO2 (+H2O) = H+ OUTPUT**
2. **CO2 (+ H2O), Lactic acid, Ketoacids = H+ INPUT**
1. Metabolism
2. Plasma pH 7.38–7.42
3. Buffers
1. HCO3– in extracellular fluid
2. Proteins, hemoglobin, phosphates in cells
3. Phosphates, ammonia in urine
4. Renal
5. **H+ = H+ OUTPUT**
48
New cards
Acid and Base: Inputs
1. **Acid**
1. Diet (acids>>bases)
2. GI secretions
3. De novo generation during metabolism
1. CO2 (volatile acid) is the largest source; while not technically an acid, it reacts with H2O to make carbonic acid, which dissociates into H+ and HCO3-
2. But under anaerobic conditions, lactic acid is generated (nonvolatile acid, fixed acid, metabolic acid; produced from source other than carbon dioxide, not excreted by lung)
3. If insulin is low (or insulin resistance), ketosis leads to organic ketoacids (diabetic ketoacidosis)
4. **Base**
1. Few dietary or metabolic sources of bases
49
New cards
pH Homeostasis
1. **Buffers**
1. Attenuate changes in pH
2. Combine with or release H+
3. Cellular proteins, phosphate ions, hemoglobin, \n **bicarbonate**
2. **Lungs (Ventilation)**
1. Rapid response
2. Corrects 75% of disturbances; can also cause them
3. **Kidney**
1. Directly by excreting or reabsorbing H+
2. Indirectly by changing the rate at which **HCO3–**buffer is reabsorbed or excreted
3. New bicarbonate synthesis
50
New cards
Hydrogen Ions
1. Proton = H+ = H atom that has given up an electron
2. **Acid:** any substance that can release (give up) a proton
3. **Base:** a substance that can accept a proton
4. Free H+ is present in body fluids in very small concentrations
1. \[H+\] in plasma = 40 nmol/L (40 x 10-9 mol/L); roughly 1/106 of the concentration of Na+, K+, Cl-, HCO3-
5. Most H+ are bound to buffers, both inside and outside cells, so levels of **FREELY CIRCULATING** H+ are usually very low
51
New cards
Physiological pH Range
1. Physiologic range = 7.38 - 7.42
1. \[H+\] = 42 - 38 nmol/L
2. A change of 0.01 pH unit = a change of \[H+\] of 1 nmol/L
3. pHarterial = 7.4
4. pHvenous/interstitial = 7.35
5. In clinical situations
1. \[H+\] can vary from 125-20 nmol/L (pH = 6.9-7.7), but this is a life-threatening condition
52
New cards
pH Regulation (Acid-Base Homeostasis)
1. Steps
1. Buffering (Occurs in seconds-minutes)
2. Respiratory response (Occurs in 1-15 minutes)
3. Renal response (Occurs in hours-days)
4. pH change
2. ACID-BASE DISTURBANCES
1. **Acidosis**
1. Lower pH
2. Compensation
3. Renal and respiratory compensation can move pH closer to normal but may not correct the problem
2. **Alkalosis**
1. Higher pH
2. Compensation
3. Renal and respiratory compensation can move pH closer to normal but may not correct the problem
53
New cards
Buffer
1. A buffer is a substance (typically a weak acid) that can reversibly bind hydrogen ions (H+)
1. Bind or release H+
2. Buffer systems prevent large changes in pH during transient accumulation of acid and bases
1. They do not eliminate acid or base equivalents
3. Blood
1. Acid load is buffered
2. Prevents significant change in pH
4. Water
1. Acid load not buffered
2. pH drops rapidly
54
New cards
Strong Acid
1. Buffer
2. HCl
1. Strong acid
2. Almost completely dissociates
1. Producing H+
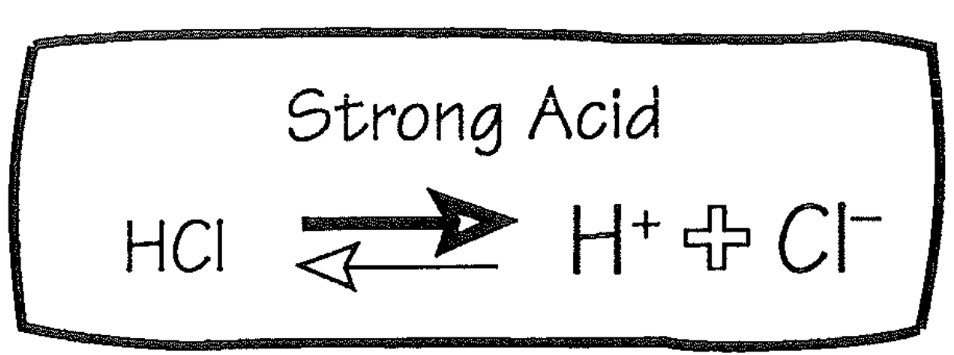
55
New cards
Weak Acid
1. Buffer
2. H2PO4
1. Weak acid
2. Only partially dissociates, yielding less free H+ than an equimolar amount of HCl
3. HPO4 can act as a buffer
3. **Weak acids are the principal buffers in the body**
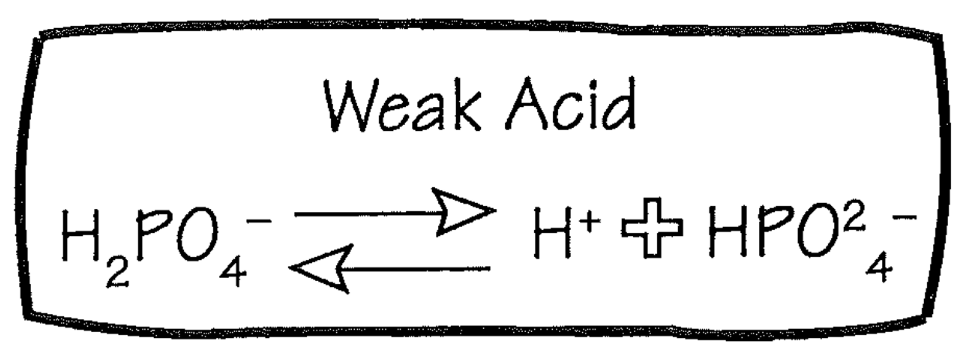
56
New cards
Intracellular Buffers
1. Majority (60%) of buffering occurs intracellularly
1. H+ exchanges with K+
2. Most important intracellular buffers are proteins, including **hemoglobin**
1. H+ will influence affinity of hemoglobin for O2
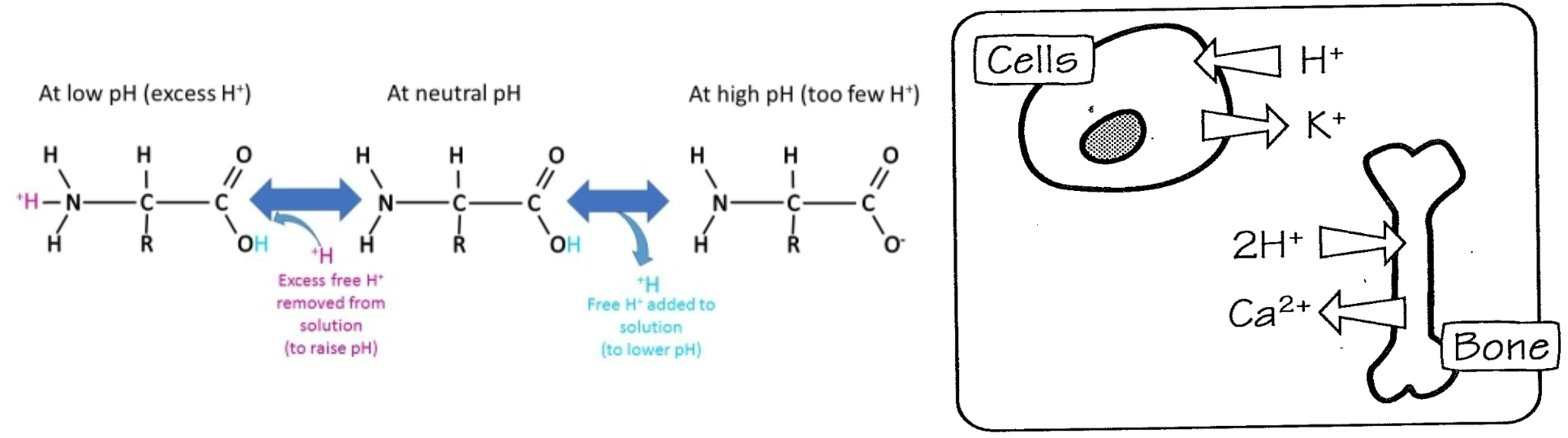
57
New cards
Carbonic Anhydrase
1. Enzyme that catalyzes the interconversion of carbon dioxide (CO2) and water (H2O) into carbonic acid (H2CO3)
1. **Carbonic acid** dissociates into H+ and HCO3
2. Family of enzymes (at least 13 mammalian); found in
1. Gastric mucosa
2. Renal tubules (lumen and intracellular)
3. Red blood cells (not plasma)
3. Very fast; rate is limited by diffusion of substrates (104-106 reactions per second)
58
New cards
CO2 Bicarbonate System
1. Concentrations of CO2 and bicarbonate are regulated **independently**
2. Because concentrations are regulated; **the ratio is regulated – pH**
3. Carbonic acid is at very low levels (3 umol/L)
1. Any carbonic acid used is replaced by infinite supply of CO2
4. Plasma HCO3- is about 600,000 X plasma H+ (24 mEq/L vs. 0.00004 mEq/L)
1. Although created in 1:1 ratio, there is significant intracellular buffering of H+
59
New cards
Henderson-Hasselbalch Equation
1. Essentially impossible to measure H2CO3 because of this rapid conversion to CO2 and H2O
2. However, easy to measure PCO2 and PCO2= H2CO3 so substitute PCO2 for H2CO3
3. CHECK PICTURE FOR
1. Solubility constant for CO2 in plasma
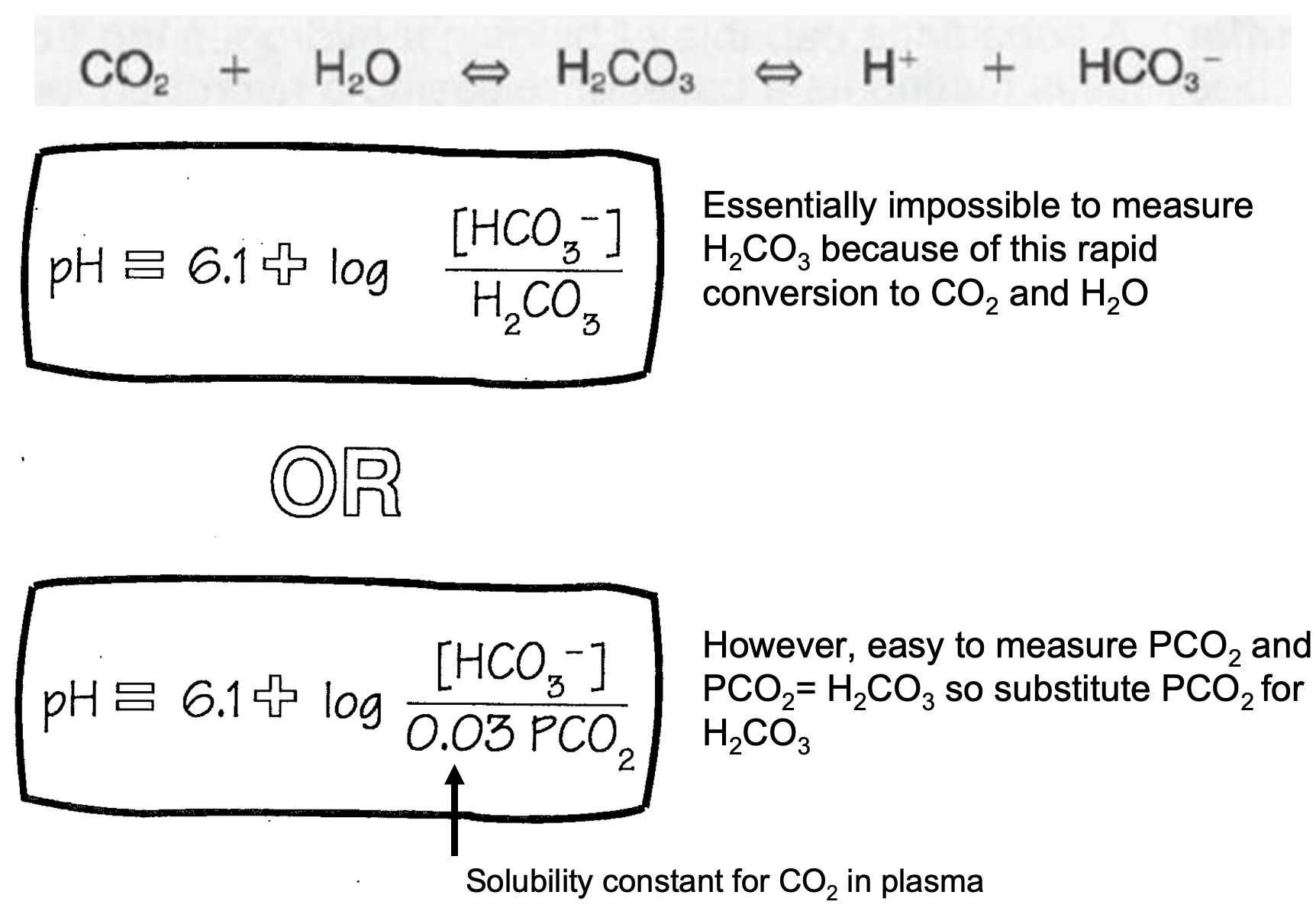
60
New cards
Law of Mass Action
1. If HCO3- + H+ are in relative excess
1. Net reaction to the LEFT
2. If CO2 is in relative excess
1. Net reaction to the RIGHT
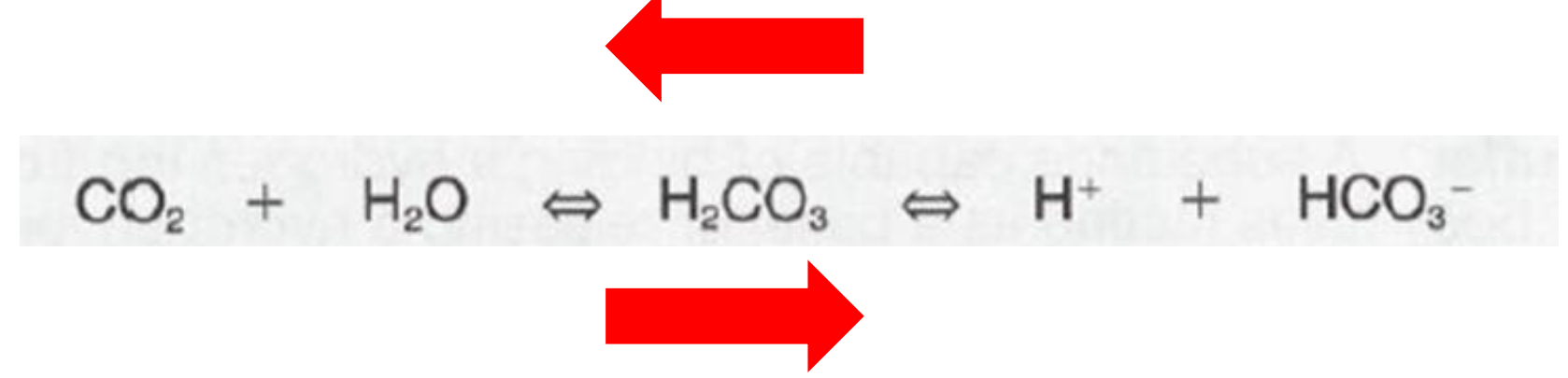
61
New cards
Extracellular Buffers
1. Amonia and Phosphate
1. Limited capacity due to buildup of end products
1. Recall law of mass action
2. **Bicarbonate**
1. **Unlimited capacity due to removal of end products**
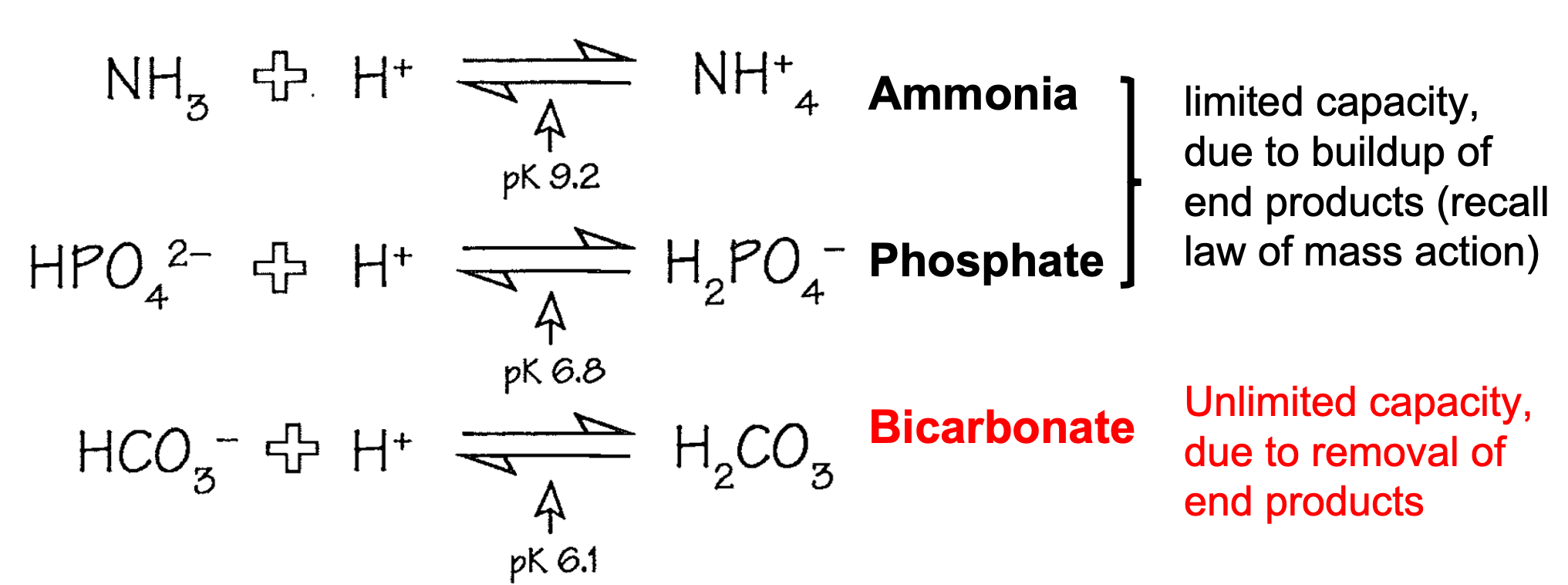
62
New cards
Bicarbonate
1. Extracellular buffer
2. H2CO3 does NOT build up
1. Instead it is converted to CO2 and H2O and dissipated
3. Steps
1. H+ and HCO3- = H2CO3
2. Turns into Carbonic Anhydrase
3. CO2 is exhaled by lungs
4. H2O is diluted into the body’s pool
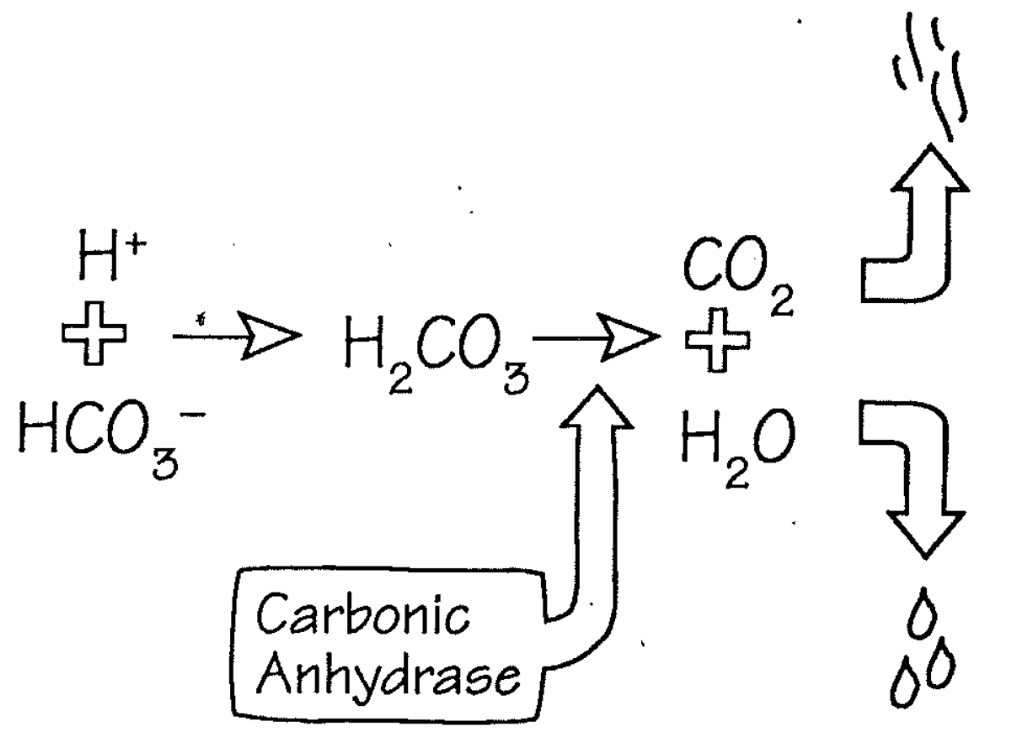
63
New cards
Acid I/O Alters Bicarbonate but not pCO2
1. When hydrogen ion levels increase, the concentration of carbonic acid rises, it dissociates into CO2 and water
1. CO2 is exhaled, restoring concentration of CO2 and carbonic acid
2. **Bicarbonate is lost**
2. When hydrogen ions are lost, CO2 and water combine to generate hydrogen ion and bicarbonate
1. CO2 is replaced from store of metabolic CO2
2. **Bicarbonate is gained**
3. **Maintaining hydrogen ion balance is therefore a** \n **problem of maintaining bicarbonate balance**
1. **For every hydrogen added to the body, one bicarbonate is lost**
2. **To maintain balance, a new bicarbonate must be** \n **generated by the kidneys**
64
New cards
CO2 and Bicarbonate
1. Input and output of CO2 and bicarbonate are independent
1. One cannot be excreted as the other
2. If there is excess generation of CO2 (increased metabolism not matched by increased ventilation), CO2 cannot be converted to fixed acid and excreted
3. Conversely, input of fixed acid cannot be converted to CO2
1. A fixed acid can release H+ (buffered), but the remaining molecule must be eliminated by the kidney
4. Every proton derived from a fixed acid that combines with bicarbonate to form CO2 ‘removes’ a bicarbonate
1. **Although CO2 is exhaled, bicarbonate is decreased**
65
New cards
pH II: Kidney Regulation
1. Understand bicarbonate ‘reabsorption’ in the kidney
2. Understand the renal handling of increased base
3. Delineate the renal handling of increased acid
1. Phosphate
2. Ammonium
3. H+ secretion
66
New cards
Renal Handling of Acids and Bases
1. In early nephron (mostly proximal tubule), kidneys reabsorb bicarbonate and secrete acids and bases
1. The mechanism of bicarbonate reabsorption involves tubular secretion of H+ ion
1. This process is not technically reabsorption
2. Bicarbonate is filtered and combines with H+ ion (carbonic anhydrase mediated) to form H2O and CO2
1. Diffuses into cell and combines to form bicarbonate and H+ ion
2. Carbonic anhydrase
3. Overall net result = bicarbonate filtered from blood is replaced by bicarbonate from ‘reabsorption’
4. Any H+ ion that combines with bicarbonate in the lumen does NOT contribute to urinary excretion of H+, but only conservation of bicarbonate
2. In distal nephron (collecting ducts), the kidney secretes either protons (type A intercalated) or bicarbonate (type B intercalated cells)
67
New cards
Bicarbonate Reabsorption
1. NHE secretes H+
2. H+ in filtrate combines with filtered HCO3- to form CO2
3. CO2 diffuses into cell
4. CO2 comhines with water to form H+ and HCO3-
5. H+ is secreted again
6. HCO3- is reabsorbed with Na+
7. Glutamine is metabolized to ammonium ion and HCO3-
8. NH4+ is secreted and excreted
68
New cards
Renal Handling of Increased Base
1. In the addition of a base, kidneys excrete bicarbonate
1. Some filtered bicarbonate is excreted in urine
2. Secrete bicarbonate **(type B intercalated cells)**
2. **Alkalosis**
1. Type B intercalated cells in collecting duct function in alkalosis
2. HCO3– and K+ are excreted
3. H+ is reabsorbed
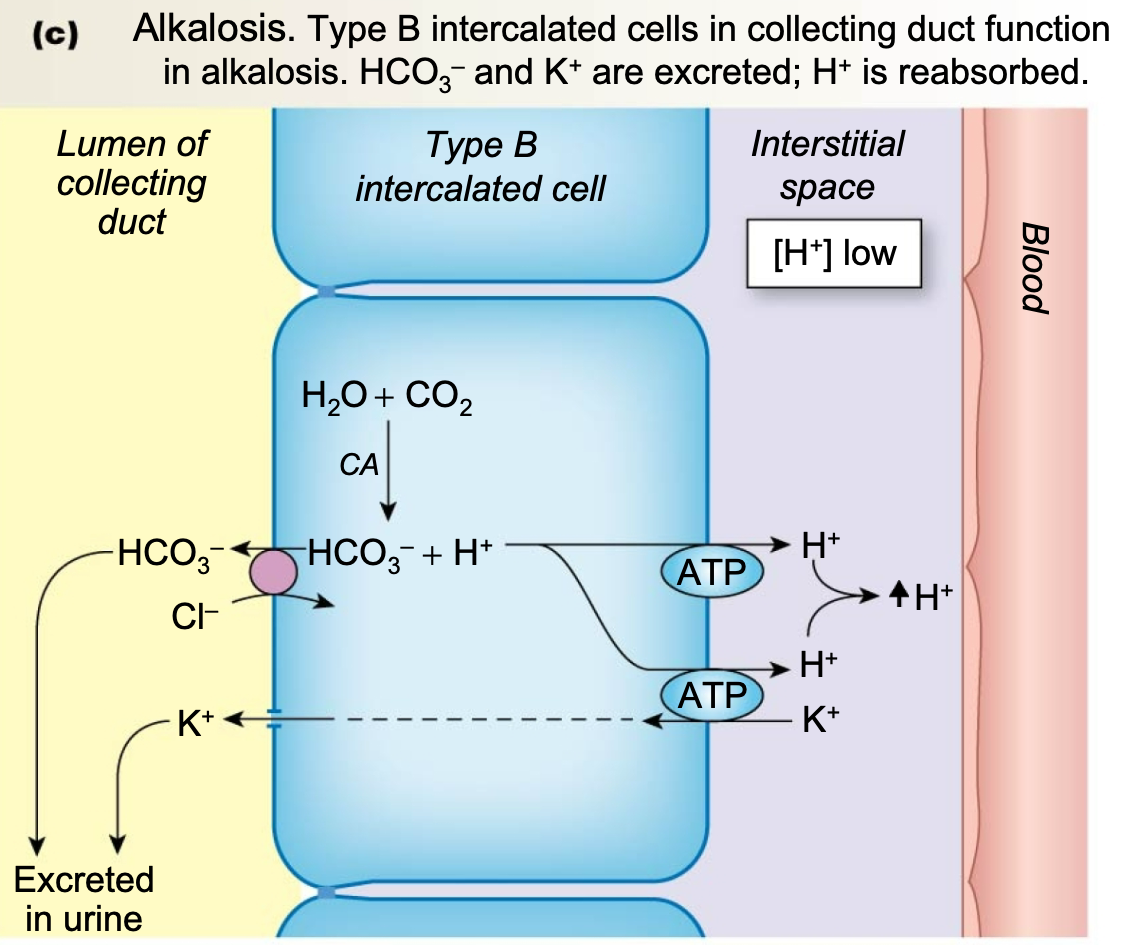
69
New cards
Renal Handling of Increased Acid
1. Since addition of acid reduces bicarbonate, kidney must replace lost bicarbonate generated from CO2 \n and water, while excreting hydrogen ion
1. **Acidosis**
1. Kidney secretes H+, which is buffered in the urine by ammonia and phosphate ions
1. Acid form is secreted
2. Reabsorbs bicarbonate to act as extracellular buffer
2. **New bicarbonate is generated (type A cells)**
1. **Cannot rely on ‘reabsorption’ of filtered bicarbonate**
3. New bicarbonate in blood is accompanied by excretion of equivalent amount of buffered hydrogen
70
New cards
Renal Handling of Increased Acid: Type A Cells
1. **Acidosis**
1. Type A intercalated cells in collecting duct function \n in acidosis
1. H+ is excreted
2. HCO3– and K+ are reabsorbed
71
New cards
Titratable Acid
1. H+ is produced from CO2 and H2O
2. H+ actively transported into lumen via H+-ATPase
3. Mainly buffered by phosphate
4. For every titratable proton, one bicarbonate is returned to blood
72
New cards
Ammonium
1. In proximal tubule, ammonium (NH4+) is generated from glutamine
2. Ammonium is transported into renal lumen in exchange for Na+ by luminal sodium/ammonium exchanger
3. Ammonium is excreted with filtered Cl- as its anion
4. **Generates new bicarbonate (via glutamine metabolism), while excreting an acid (ammonium chloride)**
5. In collecting tubule, ammonia (NH3) diffuses into lumen and combines with H+ excreted by H+ATPase
6. H+ created from CO2 and H2O by carbonic anhydrase
7. **In both cells, excretion of H+ is linked to generation of new bicarbonate**
73
New cards
Renal Transporters
1. Apical Na+H+ exchanger (NHE)
2. Basolateral Na+HCO3– symport
3. H+ATPase
4. H+K+ATPase
5. Na+NH4+ antiport
6. HCO3-Cl- exchangers
74
New cards
Summary
1. Kidneys maintain blood pH by reabsorbing bicarbonate and secreting H+
1. Urine is slightly acidic
2. Proximal tubule uses Na+H+ exchangers
1. H+ in tubular lumen is used for reabsorption of bicarbonate
2. Antiport; secondary active transport
3. Bicarbonate cannot cross the apical tubular membrane so must be converted to CO2 and H2O using carbonic anhydrase
1. Bicarbonate and H+ = carbonic acid
2. Carbonic acid +(carbonic anhydrase) = H2O + CO2
3. CO2 can cross into tubule cells, where reaction reverses and bicarbonate is synthesize
4. Excess bicarbonate (alkalosis) can be secreted in collecting duct (Type B cells) if needed
5. Distal tubule has H+ATPase pumps to increase H+ secretion
1. H+ is buffered by phosphate (filtered) and ammonium (synthesized)
2. Excretion of H+ is coupled to synthesis of bicarbonate
75
New cards
pH III: Lung & Acid-Base Disturbances
1. Delineate the respiratory response – sensors and mechanisms – to alterations in pH
2. Understand the coordinated response of the lungs and kidneys inpH regulation
3. Describe the underlying cause, and effects on pCO2, HCO3- and pH of
1. Metabolic acidosis
2. Metabolic alkalosis
3. Respiratory acidosis
4. Respiratory alkalosis
76
New cards
Respiratory Response
Increased CO2 triggers increased respiratory rate
77
New cards
Respiratory Compensation for Metabolic Acidosis
1. By law of mass action
1. **Increase in Plasma H+ and decrease in pH**
1. Carotid and aortic chemoreceptors
2. Sensory neuron
3. Respiratory control centers in medulla
4. Increase in action potentials in somatic motor neurons
5. Muscles of ventilation
6. Increase in rate and depth of breathing
7. Decrease in plasma PCO2
8. Decrease in Plasma H+ and increase in pH by law of mass action
9. Repeat from top
2. **Increase in plasma PCO2**
1. Central chemoreceptors
2. Interneuron
3. Respiratory control centers in medulla
4. Increase in action potentials in somatic motor neurons
5. Muscles of ventilation
6. Increase in rate and depth of breathing
7. Decrease in plasma PCO2
8. Repeat from top
78
New cards
Respiratory Response: pH
1. Respiratory response is limited by
1. A decrease in sensitivity as pH gets closer to 7.4
2. HCO3- availability
1. Limiting factors
79
New cards
Link Between Lung and Kidney
1. pH depends on
1. Kidney (HCO3-)
2. Lung (PCO2)
2. pH formula
1. (HCO3-)/(PCO2)
3. All disturbances of pH regulation include respiratory/metabolic components
1. One can ‘initiate’ disturbance
2. Other can compensate
4. Buffers
1. Reservoir which attenuates H+ changes protects pH), instantaneous
5. Rapid respiratory response
1. Responds to an ncrease in CO2
6. Slower renal response
1. Regulated HCO3-
80
New cards
Physiological Regulation
1. Link between lung and kidney
2. Change in either direction is small
1. Change in pH is small
81
New cards
Pathophysiology
1. Link between lung and kidney
2. Change in either direction is large
1. Change in pH is outside ability to correct
82
New cards
Acid-Base Disorders
1. Respiratory acidosis
2. Respiratory alkalosis
3. Metabolic acidosis
4. Metabolic alkalosis
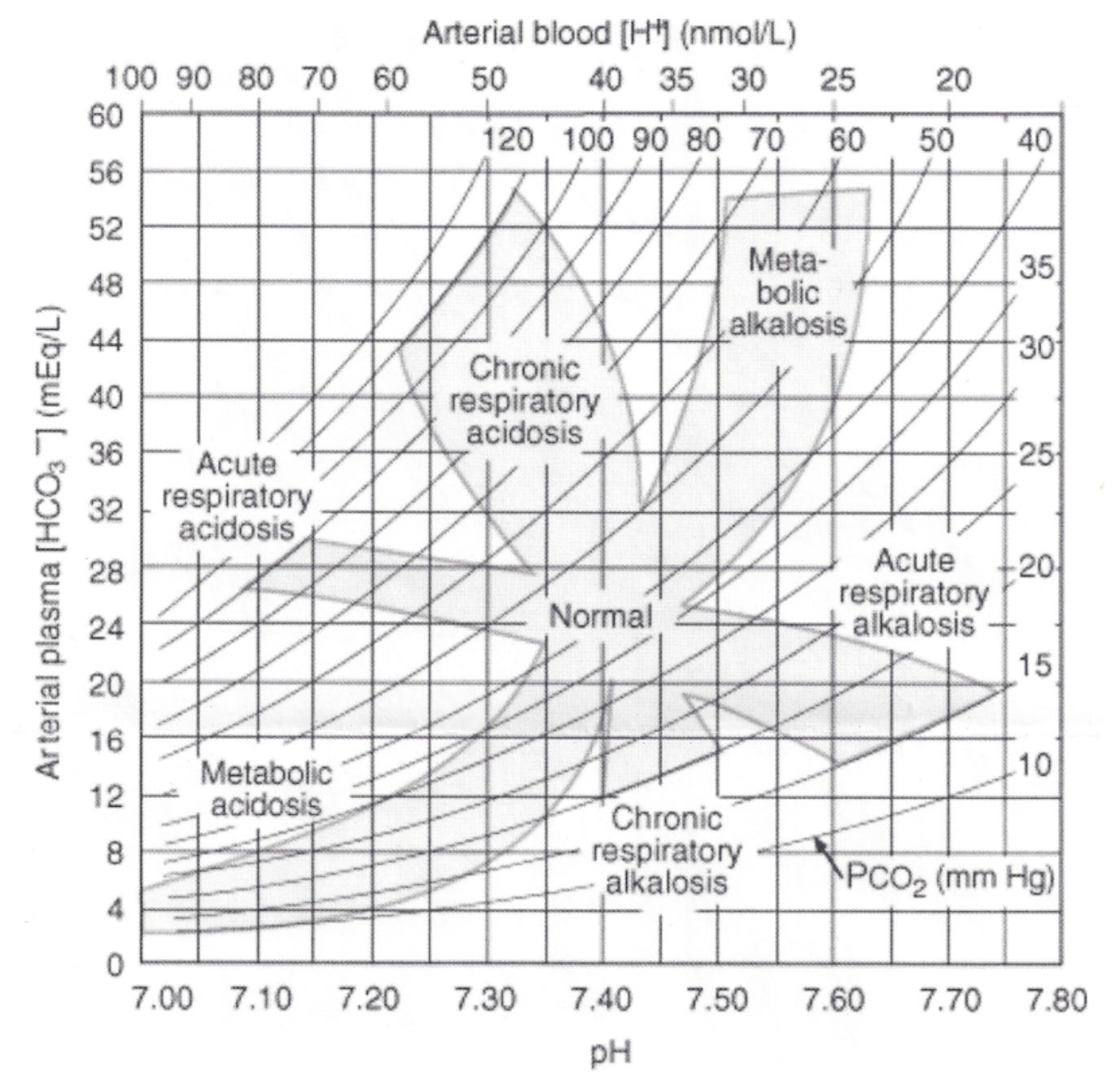
83
New cards
Respiratory Acidosis
1. When the lungs are unable to blow off CO2
1. Reaction is driven to the left
2. PCO2 is altered
3. Decreased pH
4. Increased bicarbonate
5. Kidney must excrete H+
6. Kidney must reabsorb bicarbonate
84
New cards
Respiratory Alkalosis
1. Less common than acidosis
2. When hyperventilation occurs
1. Overuse of artificial ventilation
3. PCO2 is altered (decreased)
4. Increased pH
5. Decreased bicarbonate
6. Kidney must excrete bicarbonate
7. Kidney must reabsorb H+
85
New cards
Metabolic Acidosis
1. Bicarbonate is altered
2. Decreased pH
3. Decreased bicarbonate
86
New cards
Metabolic Alkalosis
1. Bicarbonate is altered
2. Increased pH
3. Increased bicarbonate
87
New cards
Compensation
CHECK TABLE for how the body compensates for
1. Respiratory acidosis
2. Respiratory alkalosis
3. Metabolic acidosis
4. Metabolic alkalosis
1. Respiratory acidosis
2. Respiratory alkalosis
3. Metabolic acidosis
4. Metabolic alkalosis
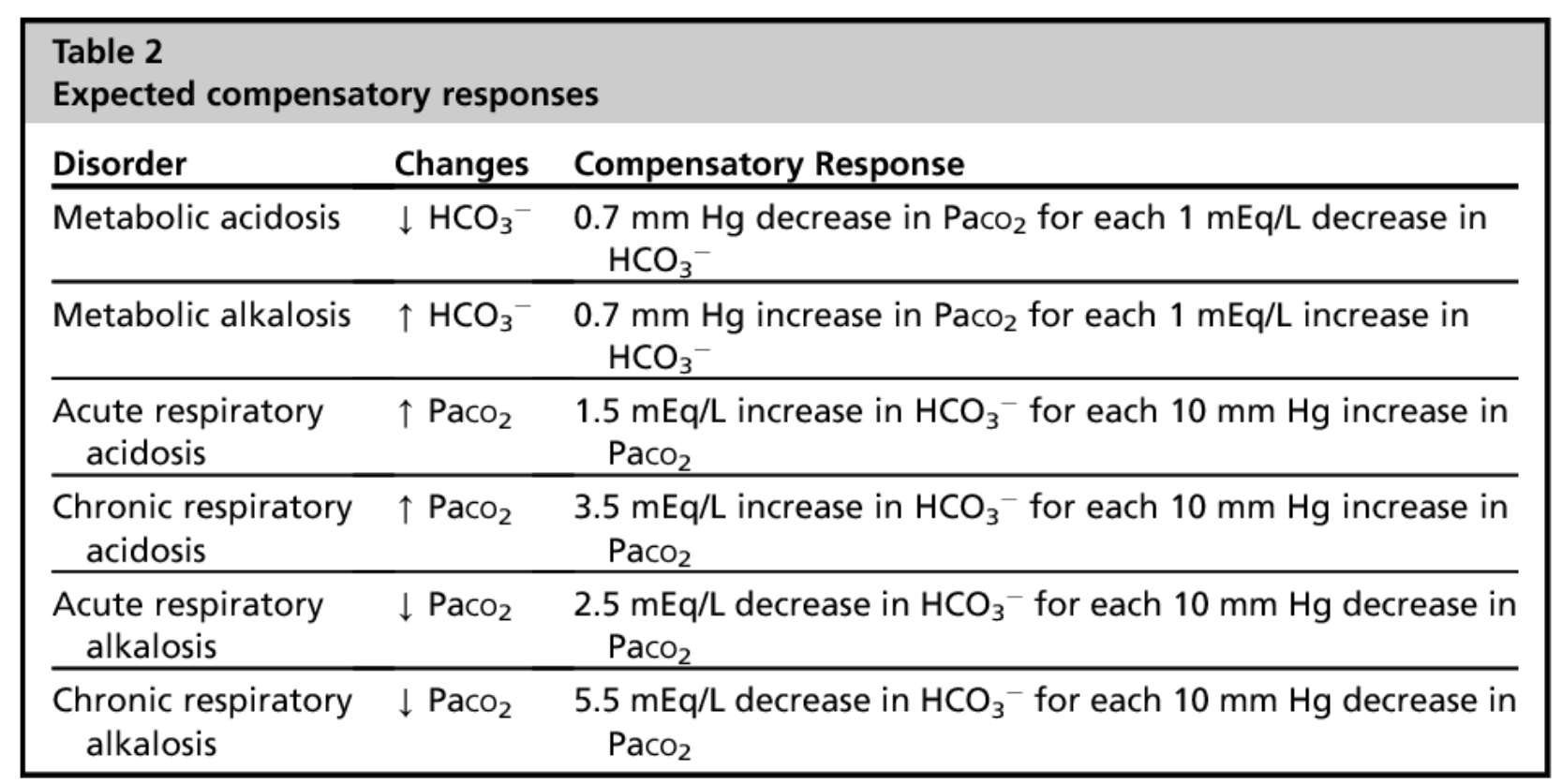
88
New cards
Kidney Compensation
1. Kidneys can compensate for respiratory \n dysfunction
2. Alkalosis
1. Less H+ is available
2. Less bicarbonate reabsorbed
3. **Extra bicarbonate secretion compensates for alkalosis**
3. Acidosis
1. Proximal tubule makes extra bicarbonate through metabolism of amino acid glutamine
2. **New bicarbonate enters blood to compensate** \n **for acidosis**
3. **Ammonia stays in urine to buffer H+**
89
New cards
Anion Gap
1. In blood chemistry, only Cl- and HCO3- are accounted for
1. Pr- and HPO42- are routinely ignored
2. “Normal anion gap” =
1. \[Na+\] – (\[Cl-\] + \[HCO3-\]) = unmeasured anions
3. In reality
1. \[Na+\] + \[unmeasured cations\] – (\[Cl-\] + \[HCO3-\] + \[unmeasured anions\]) = 0
4. **Increase in anion gap above “normal” (~12) indicates unmeasured anions**
1. **Due to**
1. **Decreased bicarbonate**
2. **Increased acids produced by body**
3. **Ingestion of certain substances (methanol, aspirin)**
90
New cards
Respiratory System Functions
1. **Exchange of gases between atmosphere and blood**
1. **Brings in O2**
2. **Eliminates CO2**
2. **Homeostatic regulation of body pH via selective retention vs excretion of CO2**
3. Protection from inhaled pathogens and irritating \n substances via trapping and either expulsion or phagocytic destruction of potentially harmful substances and pathogens
4. Vocalization (phonation) by vibrations created by airpassing over vocal cords
91
New cards
The Respiratory System
1. **Ventilation (breathing)**
1. Mechanical process that moves air into and out of \n the lungs
2. **Gas exchange** between
1. Blood and lungs and
2. Blood and tissues
3. **External respiration**
1. Ventilation and gas exchange in lungs
4. **Internal respiration**
1. Oxygen utilization and gas exchange in tissues
5. **Cellular respiration**
1. Oxygen utilization by tissues to make ATP
6. Gas exchange in lungs
1. Occurs via diffusion
2. O2 concentration is higher in lungs than in blood, so O2 diffuses into blood
3. CO2 concentration in blood is higher than in lungs, so CO2 diffuses out of blood
92
New cards
PO2: Oxygen-Hemoglobin Binding
1. Partial pressure
93
New cards
Regulation of Ventilation
1. **Skeletal muscles (unlike cardiac) are NOT spontaneously active, so they must be stimulated by neurons**
2. Rhythmic pattern of contraction and relaxation of breathing muscles arises from a neural network of
1. Spontaneously discharging motor neurons from \n cerebral cortex (voluntary breathing)
2. Respiratory control centers of pons and medulla oblongata (involuntary breathing)
3. Motor neurons innervate diaphragm and other breathing muscles
1. Regulated by descending neurons from \n the brainstem (pons and medulla)
94
New cards
Control of Breathing
1. **Pons**
1. Two respiratory control centers
1. Apneustic (stimulates inspiratory neurons in medulla)
2. Pneumotaxic (antagonizes apneustic to inhibit inspiration)
2. **Medulla**
1. Regulates **intrinsic rhythmicity,** which is influenced by other factors
2. Excitatory inspiratory neurons vs. neurons which inhibit those inspiratory neurons
3. Involuntary breathing (e.g. at rest)
1. Intrinsic to medulla
4. Voluntary (“forced,” e.g. exercise)
1. Input from cerebral cortex
95
New cards
Chemoreceptors
1. Automatic control of breathing influenced by feedback from chemoreceptors
1. Monitor pH (pCO2) of blood and interstitial fluids in brain
2. Sensitivity modulated by blood pO2
2. Locations
1. Central chemoreceptors in medulla (pCO2 to H+)
2. Peripheral chemoreceptors in carotid arteries and aortic arch (CO2, H+, O2)
96
New cards
Central Chemoreceptors
1. Found in medulla
2. Increases ventilation
1. Senses increased H+ in interstitial fluid which is caused by increased CO2 levels
2. This is not directly due to changes in plasma pH
1. H+ cannot cross blood-brain barrier)
3. Ventilation takes longer than peripheral chemoreceptors, but is responsible for 70-80% of increased ventilation
97
New cards
Peripheral Chemoreceptors
1. Found in carotid arteries and aortic arch
2. Aortic and carotid bodies respond to rise in H+ (decreased pH) due to increased pCO2, as well decreased pH independent of its effect on blood CO2
3. Respond faster than central chemoreceptors
4. **Effect of Blood PO2 on Ventilation**
1. Indirectly affects ventilation by affecting chemoreceptor sensitivity to PCO2
2. Low blood O2 makes carotid bodies more sensitive to CO2
3. Hypoxic drive
1. Carotid bodies respond directly to low oxygen dissolved in plasma (below 70 mmHg)
4. **Major control by peripheral chemoreceptors is achieved by monitoring both CO2 and O2**
98
New cards
Gas Exchange
1. Gas exchange between atmosphere and lungs upon inhalation
2. Gas exchange between lungs and blood
1. Oxygen enters blood at alveolar-capillary interface
3. Transport of gases in blood
1. Oxygen is transported in blood dissolved in plasma \n or bound to hemoglobin inside RBCs
4. Gas exchange between blood and cells
1. Oxygen diffuses into cells
5. Gas exchange between blood and cells
1. CO2 diffuses out of cells
6. Transport of gases in blood
1. CO2 is transported dissolved, bound to hemoglobin, or as HCO3-
7. Gas exchange between lungs and blood
1. CO2 enters alveoli at alveolar-capillary interface
8. Gas exchange between atmosphere and lungs upon exhalation
9. During this process, gases diffuse down concentration gradients
99
New cards
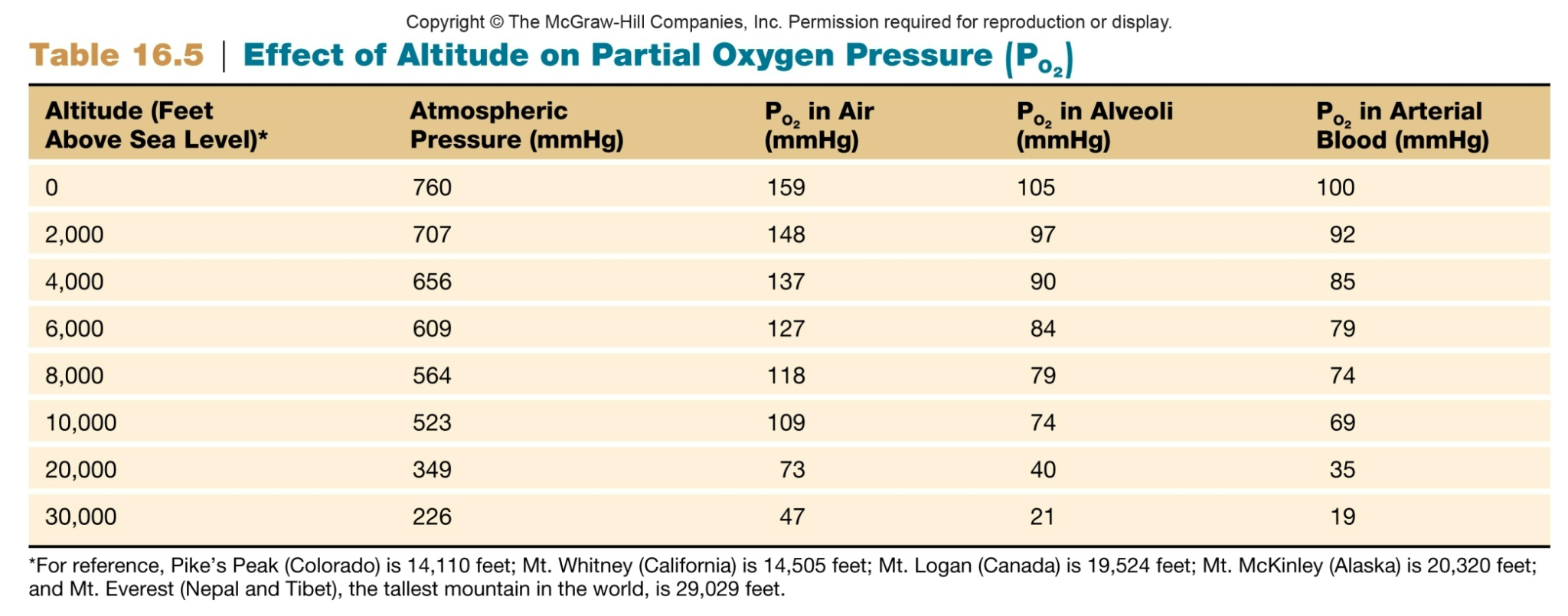
Causes of Low Alveolar PO2
1. Inspired air has abnormally low oxygen content
1. Altitude is a major factor that influences atmospheric oxygen
2. As altitude increases, TOTAL atmospheric pressure decreases
3. As altitude decreases, TOTAL atmospheric pressure increases
2. Alveolar ventilation is inadequate
1. **Decreased lung compliance**
1. Fibrotic, restrictive pulmonary diseases, lack of surfactant
2. **Increased airway resistance**
1. Narrowing/obstruction by mucus, bronchoconstriction
3. **CNS depression**
1. Slows breathing rate, decreases depth of breathing
1. Alcohol poisoning
2. Drug overdose
100
New cards
Partial Pressure Oxygen
Effect of altitude on partial oxygen pressure (PO2)