Tutorial #19: Energy II- Cellular Respiration (Krebs Cycle and Electron Transport Chain)
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
29 Terms
krebs cycle
located in mitochondria
(if O2 is present) second stage of cellular respiration
- electrons are moved from acetyl CoA and these electrons reduce more NAD+ along with FAD--> FADH2
& ATP is produced by substrate-level phosphorylation
what molecule is pyruvate converted to before entering krebs cycle?
acetyl CoA
net products of acetyl CoA transformation reactions per molecule of glucose
6 NADH, 2 FADH2, 2 ATP, 4 CO2 (2 turns of cycle)
substrate-level phosphorylation
The formation of ATP by directly transferring a phosphate group to ADP from an intermediate substrate in catabolism.
- used in both glycolysis & Krebs Cycle
How many times does the Krebs cycle turn per molecule of glucose that enters glycolysis?
2 because two pyruvates made from glycolysis
In the Krebs Cycle, electrons are removed from acetyl CoA, what happens to these electrons?
they reduce more NAD+ & FAD
how many CO2 molecules are produced during the 3 steps (glycolysis, acetyl CoA transformation reactions, & Krebs Cycle)?
6
why is the number of CO2 produced during the steps significant?
represents complete oxidation of glucose (6 carbons removed from 6 carbons in glucose)
- energy extracted and electrons transferred to NAD+ or FAD
- links metabolism to breathing (CO2 as waste product of metabolism-- lungs remove it)
where do the CO2 molecules go?
leaves the mitochondria --> cytosol --> bloodstream & delivered to lungs to be diffused out
electron transport chain (ETC)
uses the high-energy electrons from the Krebs cycle (moving H+ atoms) to create a charge differential used to synthesize ATP

charge differential (voltage)
in cellular respiration, a potential difference in charge across the inner mitochondrial matrix
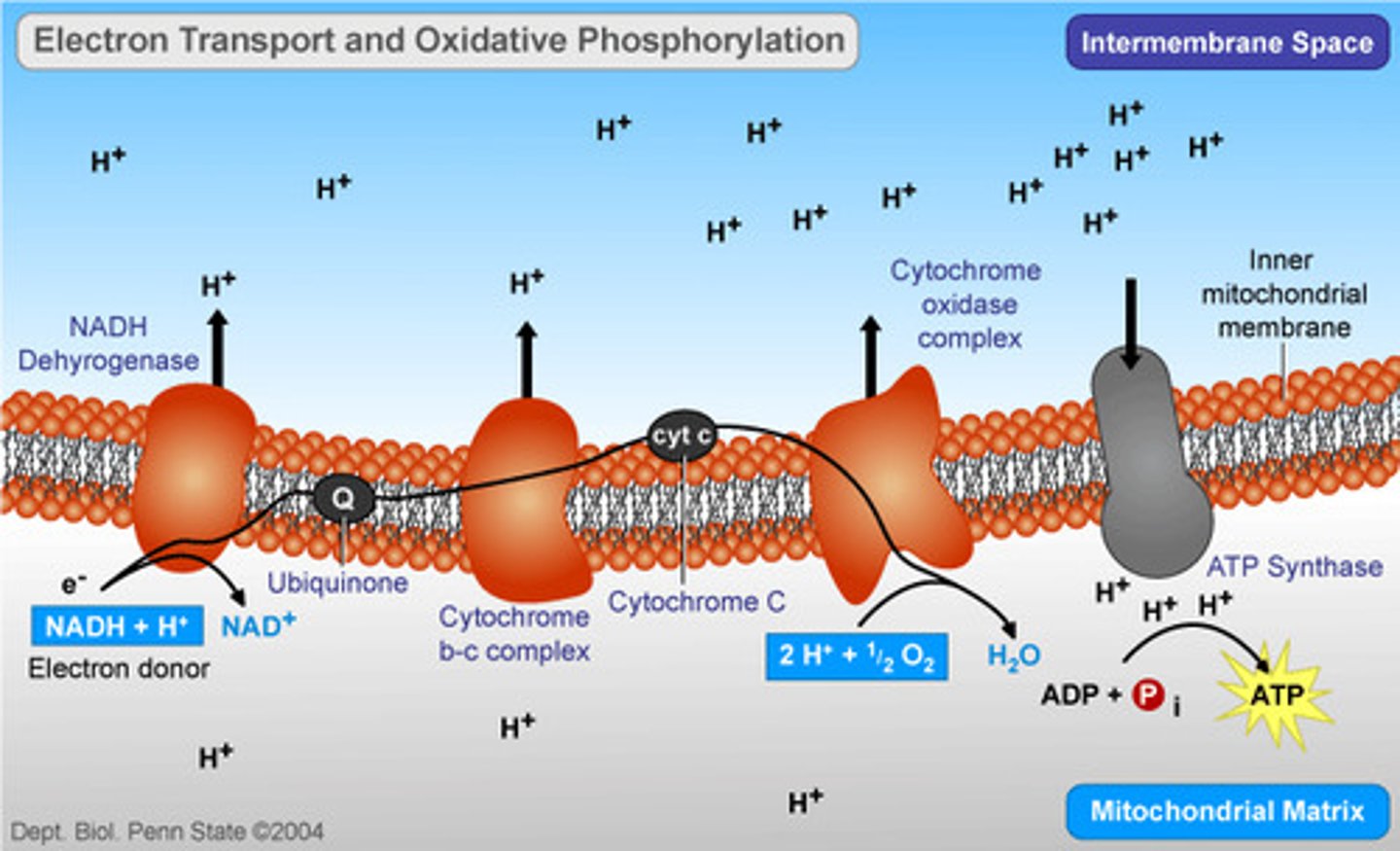
what are the roles of NADH & FADH2 in the electron transport chain
convey their electrons to the electron transport chain
The ETC is composed of a series of proteins embedded in the inner mitochondrial membrane. How do the electronegativities of the proteins differ and why is this significant?
The more electronegative the molecule = more energy required to keep electrons away from it (has high affinity for electrons)
- Electronegativity increases down chain so early carriers are less EN w/ low affinity for electrons = downhill movement is energetically favorable (-ΔG)
how does the energy of an electron change as it moves through the electron transport chain?
downhill energy (steady decrease)
- electrons starts with high energy --> low energy
this pumps the protons to make gradients + drive ATP synthase
Energy is used to pump protons across the inner mitochondrial membrane. Where does this energy come from?
from the energy released as electrons flow to more electronegative carriers (electron transfers release energy)
What is the terminal electron acceptor at the end of the electron transport chain?
oxygen
how does the proton gradient contribute to ATP synthesis?
proton gradient creates a charge differential (voltage) within inside of mitochondrial membrane---(High H+ in membrane, (low H+ in matrix---protons flow from high to low) protons buildup and create stored energy which excess protons will be used to phosphorylate ADP to synthesize ATP
Chemiosmosis
the use of energy in a H+ gradient to drive cellular work in presence of protein: ATP synthase (the movement of ions across a semipermeable membrane (in the case of cell respiration, the movement of electrons across the inner mitochondrial membrane to generate ATP)
oxidative phosphorylation
When energy is released at each step of the chain is stored in a form the mitochondrion can use to make ATP --> generates a large amount of ATP compared to substrate-level phosphorylation
how does the amount of ATP generated during oxidative phosphorylation compare to substrate-level phosphorylation?
oxidative: max = 38 ATP per glucose
substrate level: 4 (2 from glycolysis + 2 from Krebs)
why does oxidative phosphorylation generate so much more ATP?
uses a huge energy source: electron carriers (many NADH + FADH2 are produced per glucose = many ATPs); uses all electrons from glucose oxidation
substrate-level phosphorylation only occurs at glycolysis and citric acid cycle
Where does the pyruvate produced from glycolysis go if oxygen is present? How is pyruvate modified?
glycolysis occurs in cytoplasm --> produces 2 pyruvates
when O2 is present (in eukaryotic cells) pyruvate enters mitochondria and is converted to acetyl-coA ..this reduces NAD+ --> NADH and CO2 per acetyl-coA
Acetyl coA is what enters Krebs Cycle
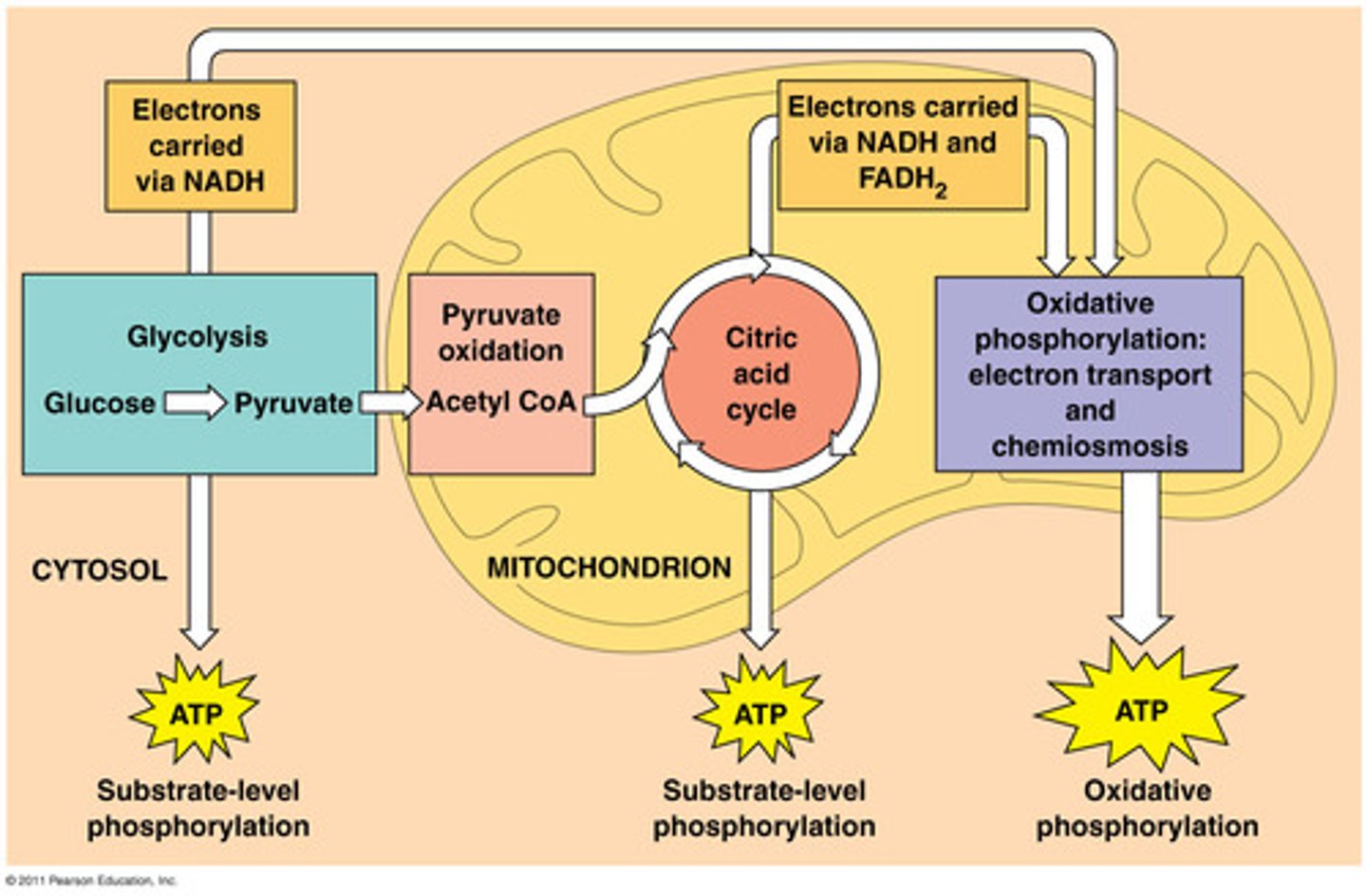
the krebs cycle converts the energy in acetyl coA to energy in _______, ________, and ______.
NADH, FADH2, and ATP
how are electrons transferred from the Krebs Cycle for use in the electron transport chain?
reduction of NAD+ and FAD
How do we finally get a big payoff of usable energy from the glucose molecule? Explain.
the ETC is a series of molecules embedded in the inner membrane of the mitochondrion, each becoming more successively electronegative until the electrons are passed to oxygen, reducing it to H2O. Energy is released from the electrons as they move down the chain that energy is used to create a proton gradient..H+ powers production of ATP
what effect would an absence of oxygen have on the electron transport chain?
O2 is needed to "pull" electrons through the ETC; without O2 the energy stored in NADH and FADH2 cannot be released to power the production of ATP through creation of proton/H+ gradient
mitochondria are enclosed by ____ membranes, each with a __________ with a unique collection of embedded proteins.
1. 2
2. phospholipid bilayer
The ______ is highly folded and contains embedded protein molecules that function in ___ synthesis. The folds called cristae increase the membranes surface area enhancing the mitochondria's ability to produce ATP.
1. inner membrane
2. ATP
what cellular conditions favor increased activity of the electron transport chain and chemiosmosis?
High ADP concentrations--need to be available to make ATP