Lab 3: Simple/Fractional Distillation
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
30 Terms
What is simple distillation?
When the vapor product is immediately channeled into the condenser, where it cools and condenses back to a liquid
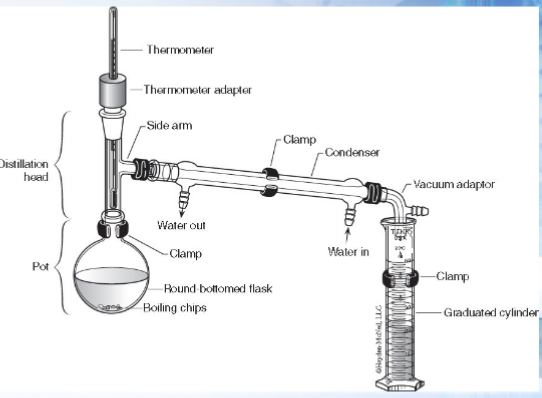
What do you absolutely need in simple distillation
a SIGNIFICANT difference in boiling point for effective purification
What is fractional distillation?
Distillation via a fractioning column (glass tube filled with either tightly packed steel wool or glass beads). There are repeated condensation and evaporation cycles during the course of distillation
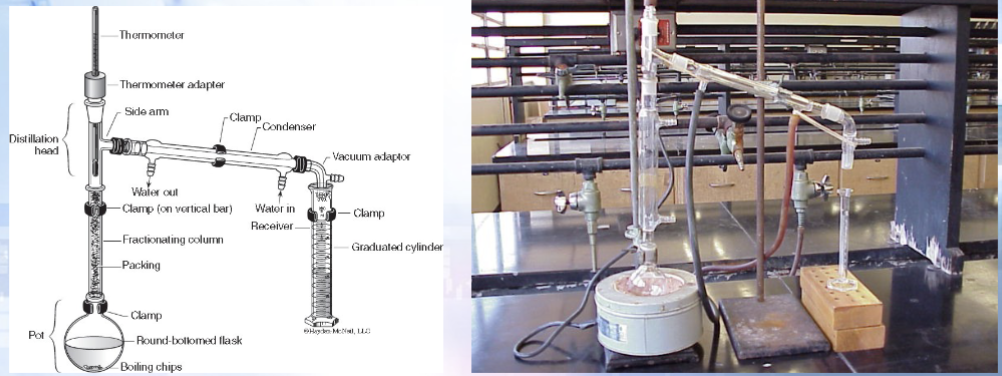
What is the difference between simple and fractional distillation? (apparatus wise)
Simple distillation involves a condenser to immediately condense the volatile liquid back to a liquid; fractional distillation uses a fractioning column
What does simple distillation do effectively?
Useful in separating miscible liquids with significantly different boiling points (larger than 100), and it is also helpful in removing a pure liquid from nonvolatile impurities
What does fractional distillation do effectively? How do you measure its effectiveness?
Helps to separate liquids with close boiling point
You measure effectiveness by column length and the density of the packing liquid
When would you use fractional distillation over simple distillation (vice versa)
Use a fractional distillation when the boiling points are less than 100 degrees Celsius apart)—more accurate and precise
Use simple distillation when the boiling point difference is greater than 100 degrees Celsius
What does the special column on the fractional distillation do?
First, the fractioning column is a glass tube filled with either packed steel wool or glass beads. It results in repeated condensation and evaporation cycles during the course of distillation
Which was does the water tubing go (in where? out where?)
The water tubing goes in the inlet and out the outlet. Inlet is closest to the end of the condenser/bottom/farthest from the distillation head. The outlet is at the top or closest to the distillation head.
What is distillation?
A purification technique used for the separation of (1) volatile miscible (will form homogenous mixture) liquids (2) volatile liquid from a nonvolatile but soluble soild
Overall, what is necessary in order to have a successful separation via distillation?
The two liquids must have a DIFFERENCE IN BOILING POINT
Is this an example of distillation: separation of pure water from a mixture of water and dissolved minerals
Yes
What is Dalton’s Law
The total vapor pressure exerted by a solution at a given temperature is equal to the sum of the partial vapor pressures of individual components
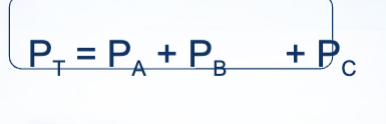
What is Raoult’s Law
Partial vapor pressure of each component in a solution is equal to the vapor pressure of the pure component multiplied by its mole fraction

What are the two types of distillation?
Simple and fractional
True or false: repeated vaporizations and condensations lead to more efficient separation/purification of the binary mixture
True: see on the graph how the benzene concentration is higher than toluene (impurity) with the second vaporization
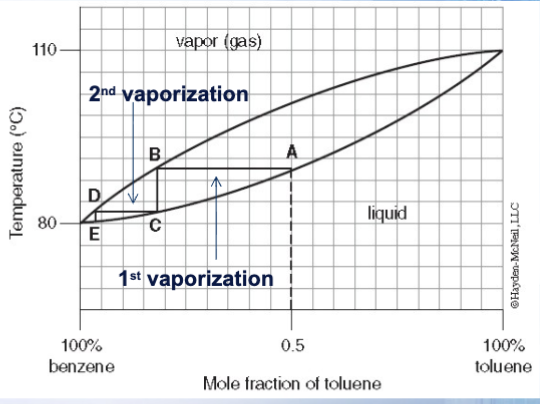
What is each separate mini distillation called?
A theoretical plate
How do you measure efficiency of fractioning column with number of theoretical plates?
The more number of theoretical plates it can generate=the more effective fractioning column
What is an azetrope?
A mixture of two or more liquids that form a constant boiling liquid of distinct proportion
ex: ethanol=mixture of 95.63% ethanol and 4.37% water (these proportions stay distinct)
What are the chemicals used in this lab?
Methanol, ethanol, and water
What experimental techniques were used?
Simple distillation and fractional distillation
What glassware was used?
Graduated cylinder, distillation head, round bottom flask, condenser, thermometer, and long-stemmed funnel
General procedure: simple distillation
Place your unknown in a round botttm flask with boiling chips
Construct your apparatus (make sure you have a beaker/graduated cylinder at the end of the condenser to collect the volatile/lower boiling point solution—will boil off sooner)
Begin boiling
Record the temperature of the first drop and then of each mL of distillate
General Procedure: Fractional Distillation
Get your unknown in a round bottom flask with boiling chips
Construct the fractional distillation apparatus (same as simple distillation with the addition of the fractioning column)—wrap the fractioning column in foil to avoid excessive heat loss
Record the temperature for the first drop and then for each mL
Collect at least 18 mL
Post lab: describe the graphs you made
Axis: temperature (y) vs volume (x) of all the data (including the first drop=your zero)
The graph connected all the points point by point and not a line of best fit
How did you determine the substance with lower boiling point? What about the one with the higher boiling point?
Once we got above 90, we stopped the experiment because we had reached our max (water), and for the fractional distillation data, the boiling point stayed around 63 degrees celsius until the big jump, signifying the transition from fully evaporating the lower boiling point component to the higher boiling point. So we assumed 63 was the boiling point .
How did you determine the percentages of your components?
For the fractional distillation data, the temperature stayed at 63 until about 4 mL, where it jumped to 91 at 5 mL. I assumed my lower boiling point component was 4 mL of my total (aka I assumed I had none leftover of my lower boiling point component). I subtracted 4 from my total to get my volume of water (the higher boiling point). I divided each of their respective volumes by the total
Sources of error
It was difficult to ensure there is a seal between the distillation head and fractioning column, so we could have lost some of our unknown, leading to a lower percent composition of this component. Also, with some of the unknown escaping instead of making it to the beaker, the large temperature jump volume point would not be representative of the mL of the unknown and the water (makes it seem like there is more water than there actually is) (still leading to a lower percent composition for methanol and a higher one for water)
Which method provided a more efficient means of separation?
Fractional (more rounds of evaporation and condensation)
Math
Maybe percent recovery (did not do it for the post lab) but percent composition (component/total) x 100