IB SL Chemistry - Topic 10
1/106
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
107 Terms
What is a Homologous Series?
A homologous series is a series of compound from the same family, with the same general formula, which differ from each other by a common structural unit (usually -CH2-)
Features of a Homologous Series (5)
Successive members of a homologous series differ by a -CH2- group
Members of a homologous series can be represented by the same general formula
Members of a homologous series contain the same functional group
Members of a homologous series have similar chemical properties
Members of a homologous series show a gradation in their physical properties
Why do members of a Homologous Series show a gradation in physical properties?
Given that successive members of a homologous series differ by a -CH2- group, they have successively longer carbon chains, and therefore successively larger molar masses
As the molar mass of a molecule increases, there are more e-, therefore more temporary dipoles and therefore, the LDF between the molecules are stronger
These stronger LDF require more energy to break, meaning successive members have increasing boiling points
What is a Functional Group?
Functional groups are the atom/group of atoms in a molecule that give it its characteristic chemical properties. It is the reactive parts of molecules.
Saturated vs. Unsaturated Compounds
Saturated: Compounds which contain only single bonds
Unsaturated: Compounds which contain double and triple bonds
What is a Hydrocarbon?
A compound consisting of only carbon and hydrogen
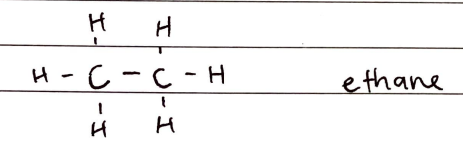
Alkanes
FORMULA***: CnH2n + 2
Prefix: N/A
Suffix: -ane
Functional Group Name: alkyl
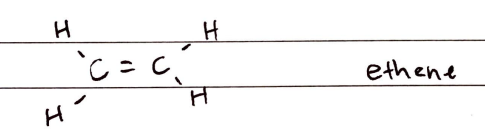
Alkenes
FORMULA***: CnH2n
Prefix: N/A
Suffix: -ene
Functional Group Name: alkenyl
Alkynes
FORMULA***: CnH2n-2
Prefix: N/A
Suffix: -yne
Functional Group Name: alkynyl
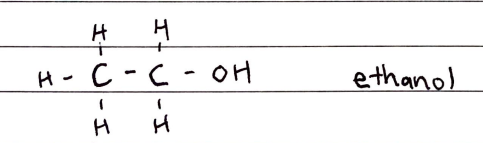
Alcohols
FORMULA***: CnH2n+1OH
Prefix: hydroxy
Suffix: -OL
Functional Group Name: hydroxyl
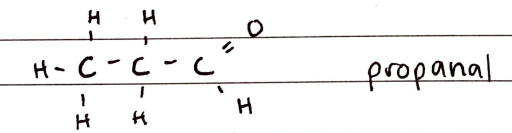
Aldehydes
FORMULA***: CnH2nO (CnH2n+1-CHO)
Prefix: formyl-
Suffix: -AL
Functional Group Name: aldehyde (formyl) (carbonyl)
Ketones
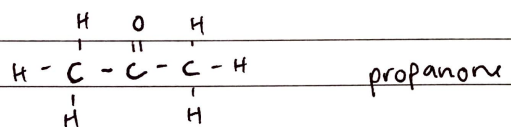
FORMULA***: CnH2nO
Prefix: oxo-
Suffix: -ONE
Functional Group Name: ketone (oxo)(carbonyl)
Carboxylic Acids
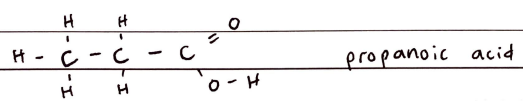
FORMULA***: CnH2nO2 (CnH2n+1COOH)
Prefix: N/A- (carboxy)
Suffix: -OIC ACID
Functional Group Name: carboxyl
Esters
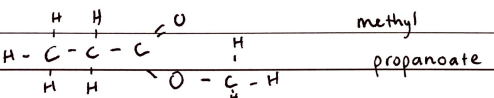
FORMULA***: N/A (R-COO-R’)
Prefix: N/A- (acyloxy)
Suffix: -OATE
Functional Group Name: carboNyl
Amines
FORMULA***: N/A
Prefix: amino
Suffix: -AMINE
Functional Group Name: amino

Amides
FORMULA***: N/A
Prefix: amido
Suffix: -AMIDE
Functional Group Name: carbox amide
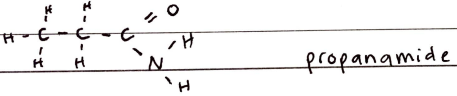
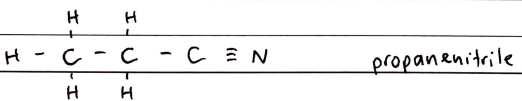
Nitriles
FORMULA***: N/A
Prefix: CYANO
Suffix: -NITRILE
Functional Group Name: nitrile
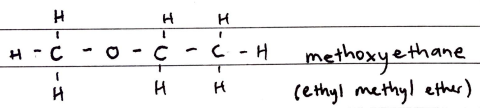
Ethers
FORMULA***: CnH2n+2O
Prefix: ALKOXY
Suffix: -ether
Functional Group Name: ethers
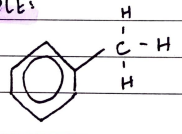
Arenes (Benzene)
FORMULA***: N/A
Prefix: PHENYL
Suffix: -benzene
Functional Group Name: benzene
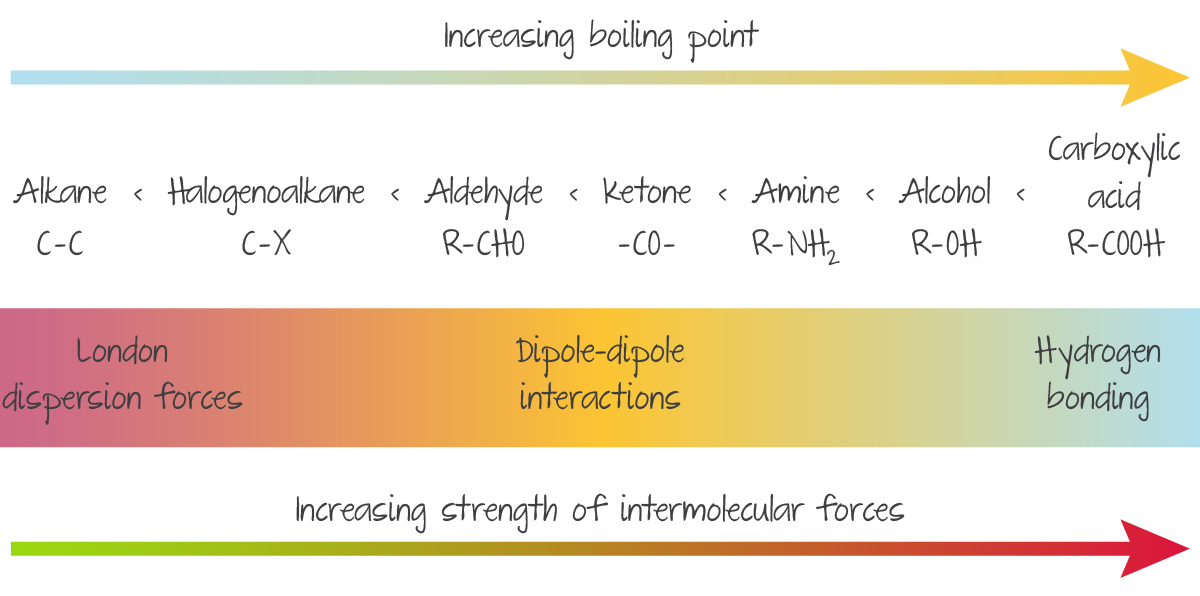
Boiling Points of Organic Compounds (lowest to highest)
Alkane, halogenoalkane, aldehyde, ketone, amine, alcohol, carboxylic acid
Molecular Formula
Actual number of atoms present in a molecule
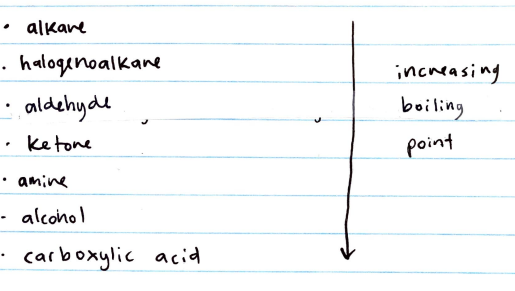
Eg) C2H6
Empirical Formula
Simplest whole number ratio of atoms in the compound
Eg) CH3
Full Structural Formula
Shows all bonds between all atoms
Condensed Structural Formula
Bonds between atoms are omitted

Skeletal Formula
All atoms are omitted leaving only the carbon backbone

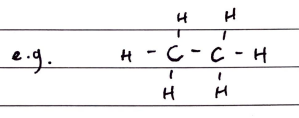
Primary, Secondary and Tertiary Compounds
PRIMARY: A primary carbon is attached to the functional group and zero or ONE other carbon atoms (2 H)
SECONDARY: A secondary carbon is attached to the functional group and TWO other carbon atoms (1 H)
TERTIARY: A tertiary carbon is attached to the functional group and THREE other carbon atoms (0 H)
Primary, Secondary and Tertiary Amines
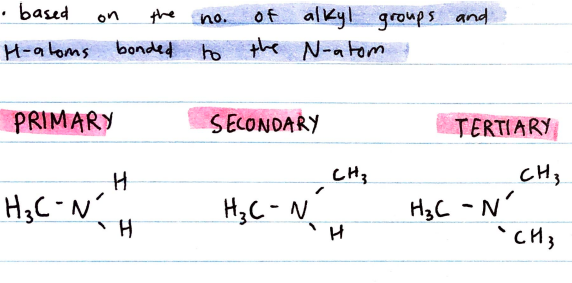
What are Structural Isomers?
Structural isomers are compounds which have the same molecular formula but different structural formulae. Different isomers have different chemical and physical properties.
Boiling points of Branched Isomers
Branched-chain isomers usually have lower boiling points than straight-chain isomers. They have lower surface area which reduces the strength of the LDFs.
Aromatic Compounds
These are compounds that have a phenyl functional group (arenes) and are known as aromatics. Aromatics are automatically classified as unsaturated compounds.
Benzene
Benzene is a cyclic structure where single bonds attach C-atoms to each other and to a H-atom
The C-atoms form 3 sigma bonds with 120º angles, making it a planar molecule
The unhybridized p-orbitals of each carbon atom overlap perpendicularly to the benzene ring, thus forming a pi electron cloud in which the e- density is concentrated above and below the plane of the benzene ring
Kekule’s Benzene Structure
Kekulé was the first to suggest a sensible structure for benzene. The carbons are arranged in a hexagon, and he suggested alternating double and single bonds between them.
It showed benzene to have alternating single and double bonds, but it was proved incorrect and instead a circle is drawn in the middle (resonance).
Chemical Evidence Going Against Kekule’s Benzene Structure
Benzene undergoes electrophilic substitution reacts and resists addition reactions
If benzene had 3 Carbon double bonds, as Kekule hypothesized, it would undergo additional reactions
However, due to the real resonance structure of benzene, addition reactions would be energetically not favoured as they would disrupt the cloud of delocalized e-
The hydrogenation of benzene is less exothermic than the hydrogenation of Kekule’s structure ((-210 K)/mol vs (-362 K)/mol)
This is because the delocalization in bbenzene reduces repulsion between e- and thus makes benzene more stable
The additional energy (152 kJ/mol) would be required to overcome the stability of the delocalization (known as the resonance energy)
Physical Evidence Against Kekule’s structure
BOND LENGTHS
All C-C in benzene are of equal lengths, intermediate between single and double bonds
If benzene contained alternating single and double bonds, we would expect the bonds to be of different lengths
Instead, bonds in benzene have a bond order of 1.5 (due to e- delocalization)
ISOMERS
Unlike Kekules structure, benzene only has one isomer of disubstituted benzene compounds (like 1,2-dibromobenzene)
This is because all of the C-C bonds are identical
If benzene contained alternating single and double bonds then we would expect 2 isomers
What is an Addition Reaction?
Occurs when two reactants combined to form a single product
Characteristic of unsaturated compounds (double bonded and triple bonded)
Involves breaking of double and/or triple bonds
What is an Elimination reaction?
Inolves splitting an organic molecule into an unsaturated hydrocarbon (alkene or alkyne) and a small inorganic molecule. (GOING FROM ANE TO ENE)

What is a Substitution reaction?
When one functional group on an organic molecule is substituted for another.
Special Types:
Aromatic Compounds (only undergo substitution)
Nucleophilic Substitution (when halogens are substituted by hydroxyl)
Free Radical Substitution (when hydrogen is substituted by a halogen)
What is an Oxidation reaction?
A reaction where a C atom makes more bonds to O and fewer bonds to H. To achieve oxidation of an organic compound, it must be mixed with an oxidizing agent in an acidic solution.
Oxidizing agents: KMnO4 (starts dark purple and turns very light pink) and Na2Cr2O7 (starts orange and turns green)
Acidic solution: H2SO4
What is a Reduction reaction?
A reaction where a C atom makes more bonds to H and/or fewer bonds to O. To achieve reduction of an organic compound, it must be mixed with a reducing agent.
Reducing agents: LiAlH4 (lithium aluminium hydride) and NaBH4 (sodium borohydride)
Fractional distillation vs. Reflux conditions
Fractional distillation (half-way oxidation or reduction - eg going from alcohol to aldehyde)
Reflux conditions (full oxidation or reduction - eg going from alcohol to carboxylic acid)
What is a Condensation reaction?
A reaction where 2 organic molecules are joined by removing a H2O molecule (water is a bi-product). (EG - esterification)
What is a Hydrolysis reaction?
Using an H2O molecule to split a large organic molecule into 2 smaller ones.
Ester + water —> carboxylic acid + alcohol
Amide + water —> carboxylic acid + amine
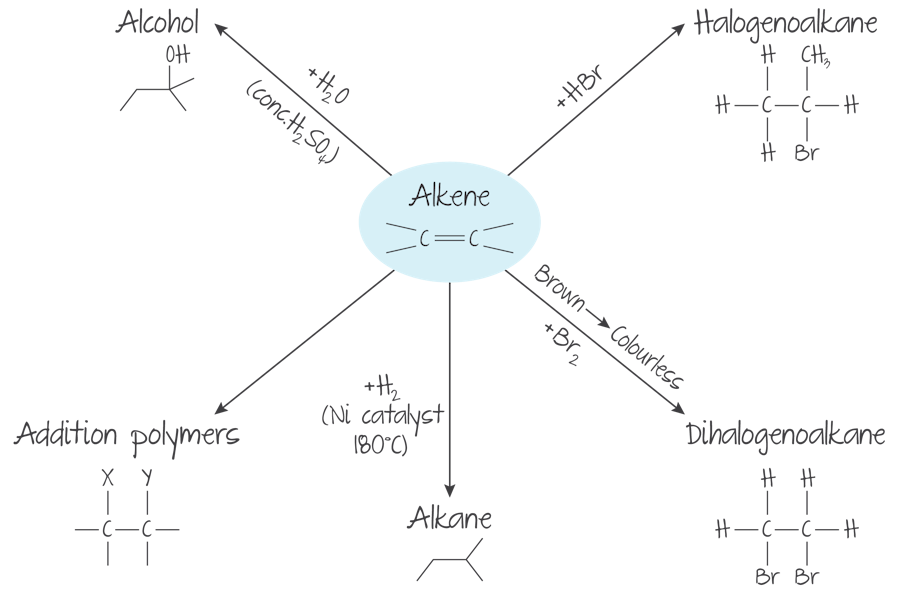
Reactivity of Alkenes
Alkenes are more reactive than alkanes due to the C=C bond
The PI bond in the C=C bond is easily broken, allowing alkenes to undergo addition reactions
Hydration of Alkenes
Alkenes react with water in the presence of an acid catalyst to produce alcohols
The acid catalyst may either be concentrated H2O4 or H3PO4
What reactions do Alkanes undergo?
Combustion, Cracking, Substitution (Halogenation)
What reactions do Alkenes undergo?
Addition reactions (halogenation, hydrogenation, hydration, hydrogen halidation, oxidation)
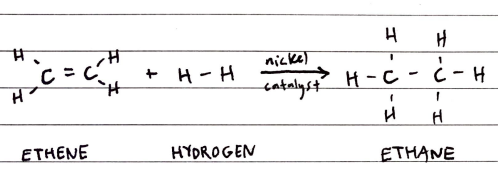
Hydrogenation of Alkenes
Alkenes react with H2 in the presence of a Nickel catalyst at about 150ºC
Halogenation of Alkenes
Alkenes react with halogens to produce dihalogeno compounds. These reactions occur readily at room temperature and are accompanied by the loss of the colour of the halogen.
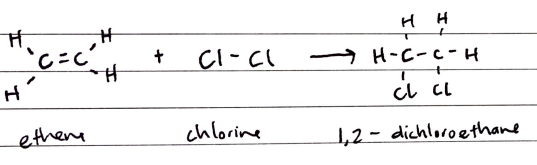
Hydrogen Halide Addition of Alkenes
Alkenes react with hydrogen halides to produce halogenoalkanes
All the hydrogen halides can react in this way but the ones with weaker bonds react more readily. Te hydrogen halide bond decreases in strength down group 17
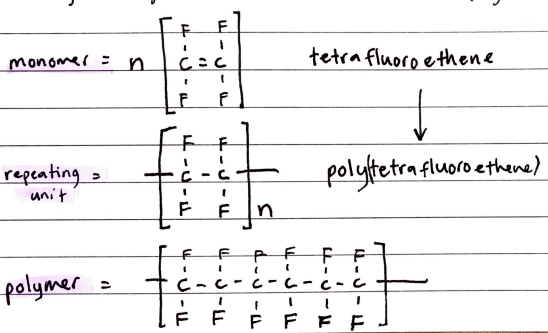
Polymerization of Alkenes
Alkenes can undergo addition polymerization to join together and form addition polymers
Economic importance of Alkene reactions
POLYMERIZATION
Synthesis of plastics which are cheap and versatile
MANUFACTURE OF MARGARINE
Oils containing unsaturated hydrocarbons are hydrogenated to form margarine
MANUFACTURE OF ALCOHOLS
Hydration of alkenes forms alcohols
Low reactivity of alkanes
The C-C and C-H bonds are non-polar bonds, so alkanes are not susceptible to attack by most reactants
The C-C and C-H bonds are strong covalent bonds, meaning that alkanes will only react in the presence of a strong enough energy source to break these bonds (EA)
What is heterolytic fission?
Occurs when a covalent bond splits into 2 oppositely charged ions (one + and one -)
THIS IS WHAT WE ARE USED TO
What is homolytic fission?
Occurs when a covalent bond breaks and each atom gets one electron
This produces 2 free radicals (highly reactive species with an unpaired electron)
What is a Free-Radical?
Highly reactive species with an unpaired electron
What is Free-Radical Substitution?
Only occurs in the presence of UV light
In this rxn, another reactant (eg. Cl) takes the place of a H atom in the alkane
This rxn required the energy from UV light to break the covalent bond in Cl2 (photochemical homolytic fission) to form Cl free radicals
What are all of the stages and steps of Free-Radical substitution?
Initiation
UV light is sued to cause the homolytic fission of the diatomic halogen. This creates 2 free radicals. [PHOTOCHEMICAL HOMOLYTIC FISSION]
Cl-Cl —> •Cl + Cl•
Propagation
Consumes a free radical, BUT also produces a free radical so the rxn can continue
•Cl +HCH3 —> HCl + •CH3
•CH3 + Cl-Cl —> ClCH3 + •Cl
Termination
Consumes 2 free radicals and produces neutral, unreactive molecules
•Cl + •Cl —> Cl2
•CH3 + •CH3 —> CH3CH3 (ethane)
•Cl + •CH3 —> ClCH3
Why would free radical substitution mechanism give a poor yield of the desired product?
This mechanism rarely results in the intended halogenoalkane because the substitution of the halogen is totally random and can happen to any hydrogen on any molecule in the rxn mixture.
Further substitutions would occur, thus giving a mixture of many different products and so a low probability of the desired product.
Bromine water unsaturation test
When Br water is added to alkenes, an addition reaction occurs. The alkene decolourizes the brown colour of the Br water to COLOURLESS (not clear******).
ALKENE = TURNS IT COLOURLESS
Alkanes do not react readily with Br water as the presence of UV light is required. Thus, when Br water is added, NO REACTION OCCURS and there is NO COLOUR CHANGE.
ALKANE = IT REMAINS BROWN
Br Water = Br(aq)
Oxidation of Alcohols
Alcohol molecules can react with oxidizing agents which selectively oxidize the ol-carbon
Eg) potassium permanganate, potassium dichromate, sodium dichromate
Oxidation of Primary Alcohols
Primary alcohols are oxidized in a two-step reaction that first produces an aldehyde which is further oxidized to a carboxylic acid
If the aldehyde is the required product, it can be removed from the rxn mixture by fractional distillation
This is possible as aldehydes have a lower boiling point than alcohols and carboxylic acids because they don’t have H-bonding between reactants
Bi-product: water
If the carboxylic acid is the required product, we must leave the aldehyde in contact with the oxidizing agents for a prolonged period of time (REFLUX)
This required heating under reflux

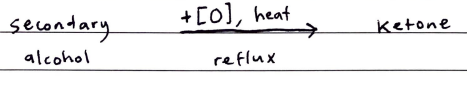
Oxidation of Secondary Alcohols
The oxidation of secondary alcohols is a one-step process that produces a KETONE
After the ketone is produced, no further oxidation is possible as there are no more H-atoms attached to the ol-carbon
Bi-product: water
Conditions for oxidation reactions of alcohols
ALDEHYDE
Acidified oxidizing agent, heat, use excess alcohol & distill immediately
CARBOXYLIC ACID
Excess acidified oxidizing agent, heat, reflux longer
KETONE
Acidified oxidizing agent, heat, reflux
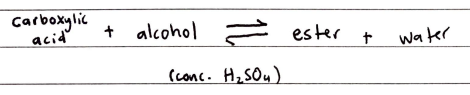
Esterification
Alcohols react with carboxylic acids to form esters in a condensation reaction
The reaction is an EQUILIBRIUM RXN and is catalyzed by conc. H2SO4
Unlike carboxylic acids and alcohols, esters CANNOT form H-bonds between molecules so they have lower boiling points
Thus, they can be seperated by distillation
They are mostly insoluble in water (form a layer on the surface)
REQUIRED HEATING UNDER REFLUX
Use of Esters (3)
Food flavouring (synthetic)
Fragrant odours for perfume
Make surfactants (eg. soap)
Combustion Reactions of Alkanes
COMPLETE COMBUSTION
Alkanes burn in the presence of excess O2 to produce CO2 gas and H2O gas
INCOMPLETE COMBUSTION
When the O2 supply is limited, the products are either CO gas and H2O gas or, when O2 is extremely limited, SOLID carbon and H2O gas
Combustion of Alcohols
Like alkanes burn in excess O2 to produce CO2 gas and H2O gas
The enthalpy of combustion of alcohols increases up the homologous series as the number of CO2 molecules produced increases
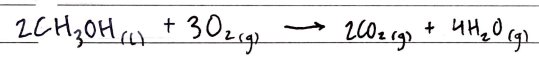
Physical Properties of AMINES
MP/BP
Primary and secondary amines have higher MP and BPs since they have H-bonds between molecules (N-H)
Longer amine chain = stronger LDF = higher melting + boiling points
SOLUBILITY
Water can form H-bonds with all amines (including teriary) meaning that all amines are SOLUBLE IN WATER
The longer the amine chains are, the less soluble they come as the chain becomes non-polar
Production of Amides
Carboxylic Acid + Amine < — > Amide + Water
It is a condensation rxn that requires conc, H2SO4 AS A CATALYST
Primary and secondary amines can undergo this rxn (not 3º)
ITS A REVERSIBLE RXN (hydrolysis can take place of amides)
Markovnikov’s Rule
When more than one product is possible, the carbon with most electron bonds receive it
“The rich get richer”
Naming Polymers
Find the MONOMER unit and name it. Add “poly” in front of that name.
Bond Strengths and Lengths
Strengths: Triple > Double > Single
Triple is most strong while single is most weak
Lengths: Single > Double > Triple
Triple is shortest while single is longest
What is a Nucleophile?
A fully negative ion or polar molecule that is attracted to the nucleus of another atom.
Eg) OH- , CN-, H2O, NH3
What is the Transition State Complex/Activated Complex?
A point in time where bonds are breaking at the same time as they are forming
What is steric interference?
It prevents or slows down a chemical reaction, caused by the arrangement of atoms in a molecule.
It makes it harder for the rxn to take place
What is SN2?
It has 2 steps involved in its RDS
It involves the formation of a transition state complex
0º, 1º and 2º experience this one (although 2º do it badly)
What is SN1?
It has 1 step involved in its RDS
It involves the formation of a carbocation
3º experience this
What is the speed of each reaction in Nucleophilic substitution?
3º>0º>1º>2º
What is Condensation Polymerization?
Condensation rxns are used to join together a series of monomers that contain 2 functional groups; WATER forms as a bi-product
POLYESTER = dioic acid + diol
POLYAMIDE = dioic acid + diamine
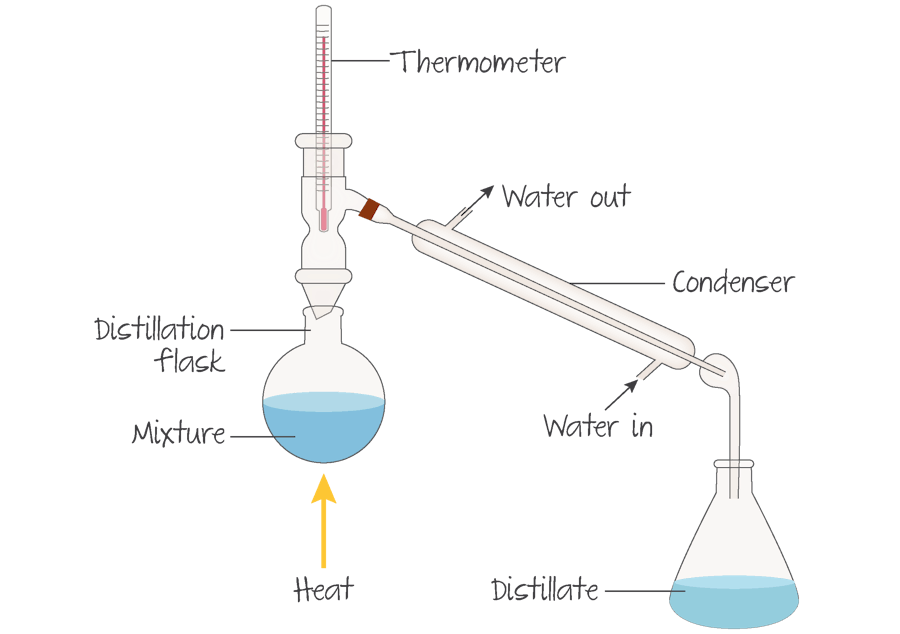
What is fractional distillation?
Fractional distillation is a process by which components in a chemical mixture are separated into different parts (called fractions) according to their different boiling points. Fractional distillation is used to purify chemicals and also to separate mixtures to obtain their components.
What is reflux?
Reflux involves heating the chemical reaction for a specific amount of time, while continually cooling the vapour produced back into liquid form, using a condenser. The vapours produced above the reaction continually undergo condensation, returning to the flask as a condensate.
What is electrophillic addition
Number of Structural Isomers:
Methane: ?
Ethane: ?
Propane: ?
Butane: ?
Pentane: ?
Hexane: ?
Heptane: ?
Octane: ?
Nonane: ?
Decane: ?
Methane: 0
Ethane: 0
Propane: 0
Butane: 2
Pentane: 3
Hexane: 5
Heptane: 9
Octane: 18
Nonane: 35
Decane: 75
Class vs. functional group (example?)
Class: alcohol
Functional group: hydroxyl
Arenes
Aromatic compounds contain a benzene ring <that forms resonance > which is known as a phenyl functional group
Why do isomers have lower BP/MPs?
The branching of a chain produces a more spherical shape to the molecule. There is less surface contact between the molecules than with straight-chain isomers. Thus, branched-chain isomers have weaker intermolecular forces and, consequently, lower boiling points.
Strength and IMF trend in functional groups
The solubility of organic compounds in water depends on their ability to ________________________________________________
The solubility of organic compounds in water depends on their ability to form hydrogen bonds with water molecules.
Hydrophobic (def)
The term hydrophobic refers to the non-polar hydrocarbon chain of a molecule which is insoluble in polar solvents such as water.
Benzene is a known ____________, so inhalation of benzene vapors is definitely not recommended.
CARCINOGEN
Carbon monoxide characteristics
Note that carbon monoxide is a highly poisonous gas, with no taste, smell or colour. It binds strongly to haemoglobin in the blood, which leads to suffocation and eventually death if it is inhaled in sufficiently high concentrations.
Incomplete combustion is characterised by…
This is known as incomplete combustion and is characterised by the appearance of a yellow or orange flame.
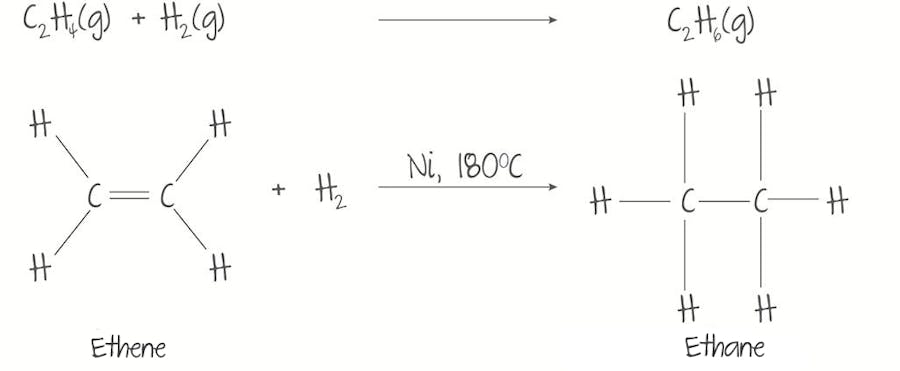
Alkenes (Hydrogenation)
Catalyst: ?
Conditions: ?
Real-life: ?
H2 IS ADDED TO CARBON DOUBLE BOND
Catalyst: Nickel (Ni) catalyst.
Conditions: High pressure and high temperature (180º)
Real-life: Maragarine production
Alkenes (Hydration)
Catalyst: ?
Conditions: ?
Real-life: ?
STEAM (H2O(g)) IS ADDED TO ALKENE
Catalyst: H2SO4
Conditions: -
Real-life: To produce alcohols
Alkenes (Halogenation)
Catalyst: ?
Conditions: ?
Real-life: ?
Alkenes react with halogens to produce dihalogenoalkanes.
Catalyst: ?
Conditions: Room temperature and are indicated by the decolourisation of the reacting halogen
Real-life: ?
Polymers real-life application
Poly(chloroethene), more generally known as PVC (polyvinyl chloride)
Construction materials, packaging and covers for electrical cables.
Poly(ethene)
Plastic bags, bowls, bottles, packaging
Alkene summary
Alcohols (Oxidation)
Catalyst: ?
Conditions: ?
Real-life: ?
Catalyst: Oxidizing Agents
Potassium manganate(VII)
Acidified potassium dichromate(VI) (Cr2O72−/H+).
Orange solution which changes to a green colour (Cr3+ ion)
Potassium manganate(VII) solution
Purple to colourless (Mn2+)
Conditions: ?
Real-life: ?
How can you tell any oxidation rxn is complete using Dichromate?
The dichromate ion forms an orange solution which changes to a green colour as it is reduced to the Cr3+ ion
If the aldehyde is the desired product, it can be removed as it forms by ______________.
Explain this process.
If the aldehyde is the desired product, it can be removed as it forms by distillation (Figure 5).
This is possible because the boiling point of the aldehyde is lower than that of both the alcohol and the carboxylic acid. As the aldehyde evaporates and rises up the distillation column, it passes through the condenser where it condenses back to a liquid and runs down into the flask.