Polarity
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
what is a Polar covalent bond?
when the two atoms electronegativity differentiates (their not sharing the electrons equally)
what is a non polar covalent bond?
When the atoms have identical electronegativity!!
What is the difference between a polar covalent bond, and an ionic bond?
Polar: electronegativity is slightly different
Ionic: very different electronegativities
Why is carbon dioxide non polar even though it's bonds are polar?
Which ones are polar covalent and which ones are non polar covalent?
n2
HF
F2
NO
FCL
Polar covalent: HF, NO, FCL
nonpolar covalent: N2 F2
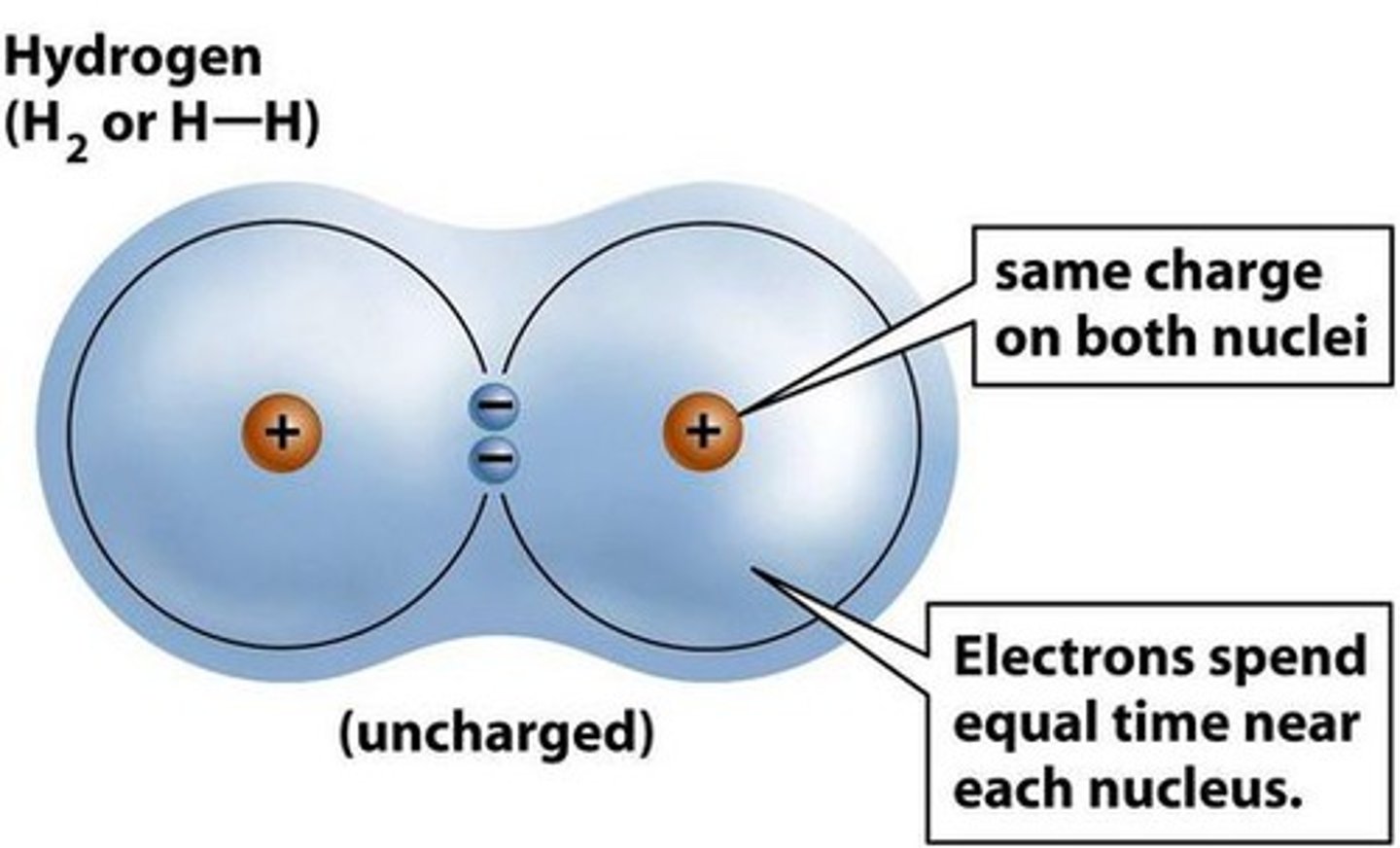
How do they form a covalent bond?
By sharing electrons
The more electronegative your molecule is?
then its the negative partial charge
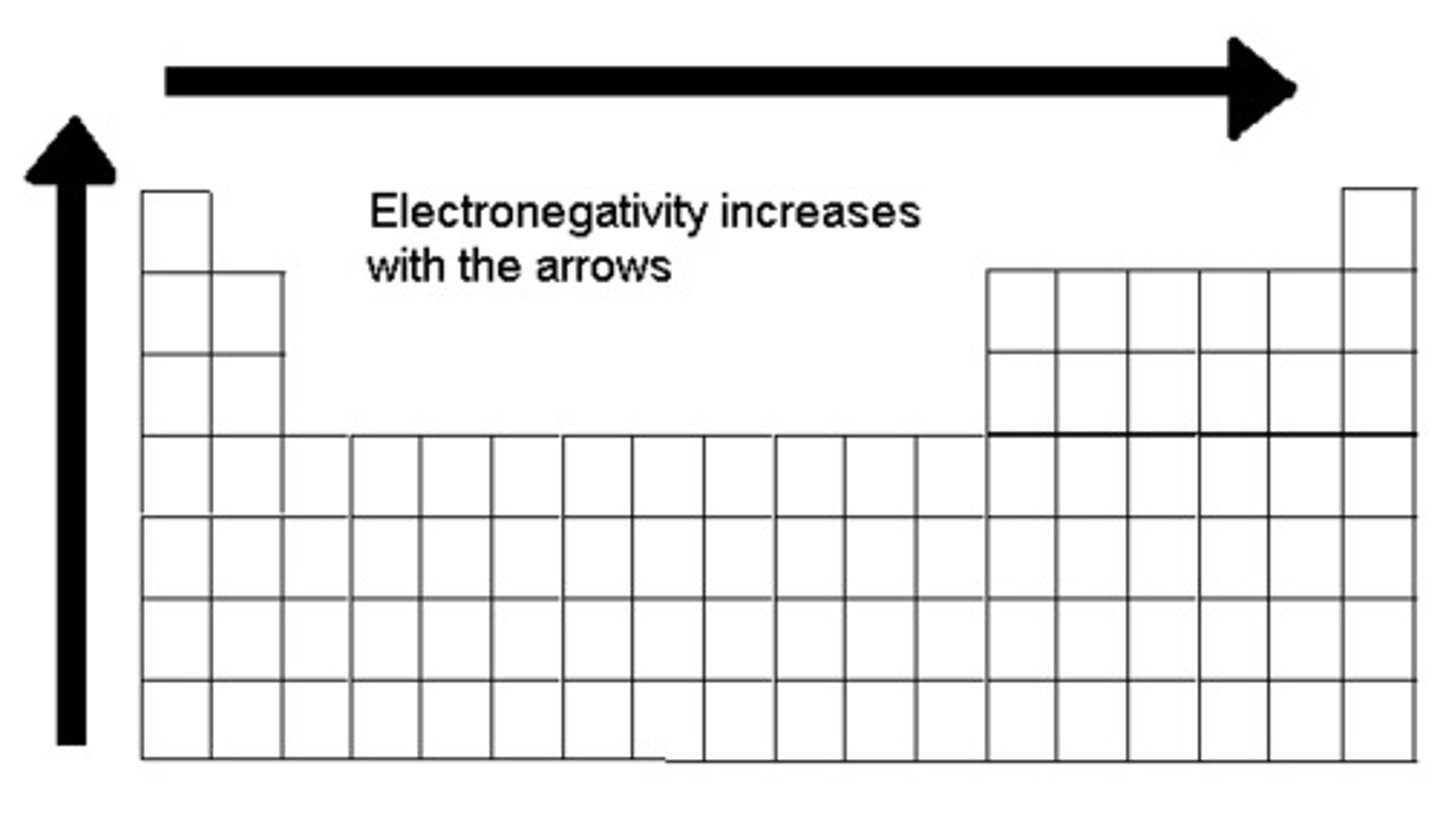
Electronegativity trend
Linear
180*
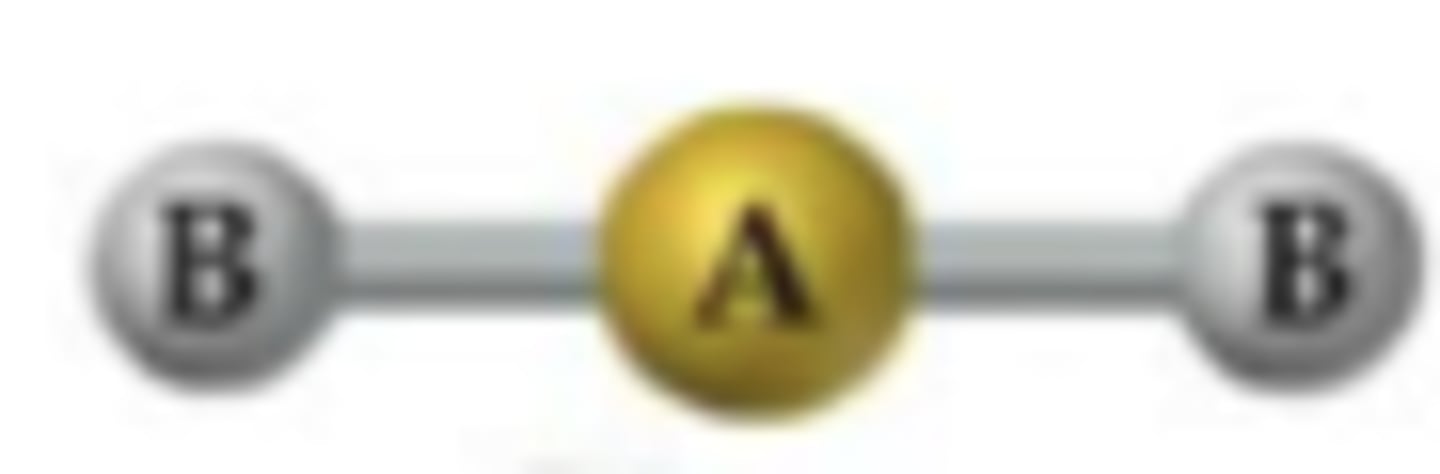
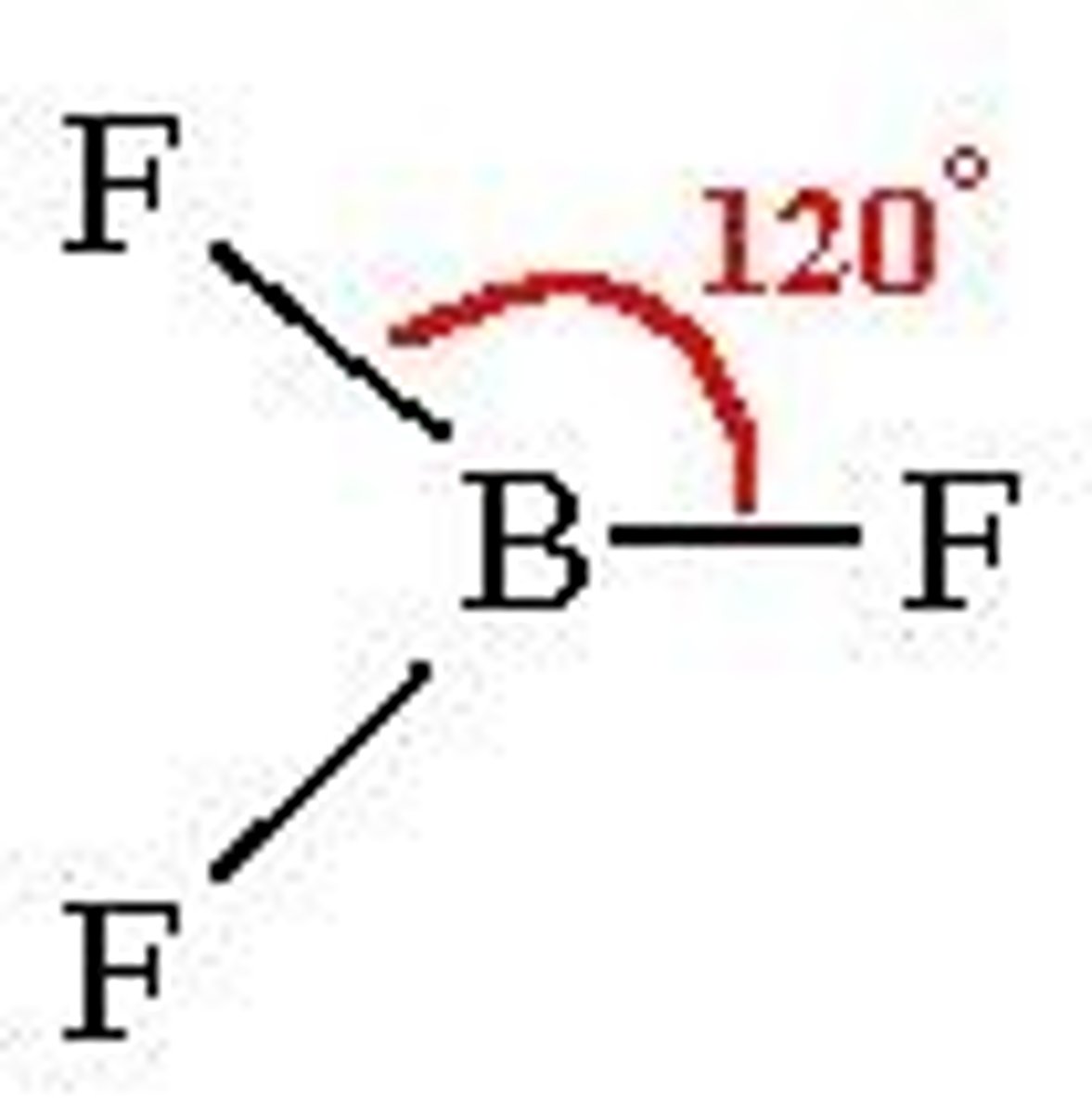
Trigonal Planar
(flat)
Tetrahedral
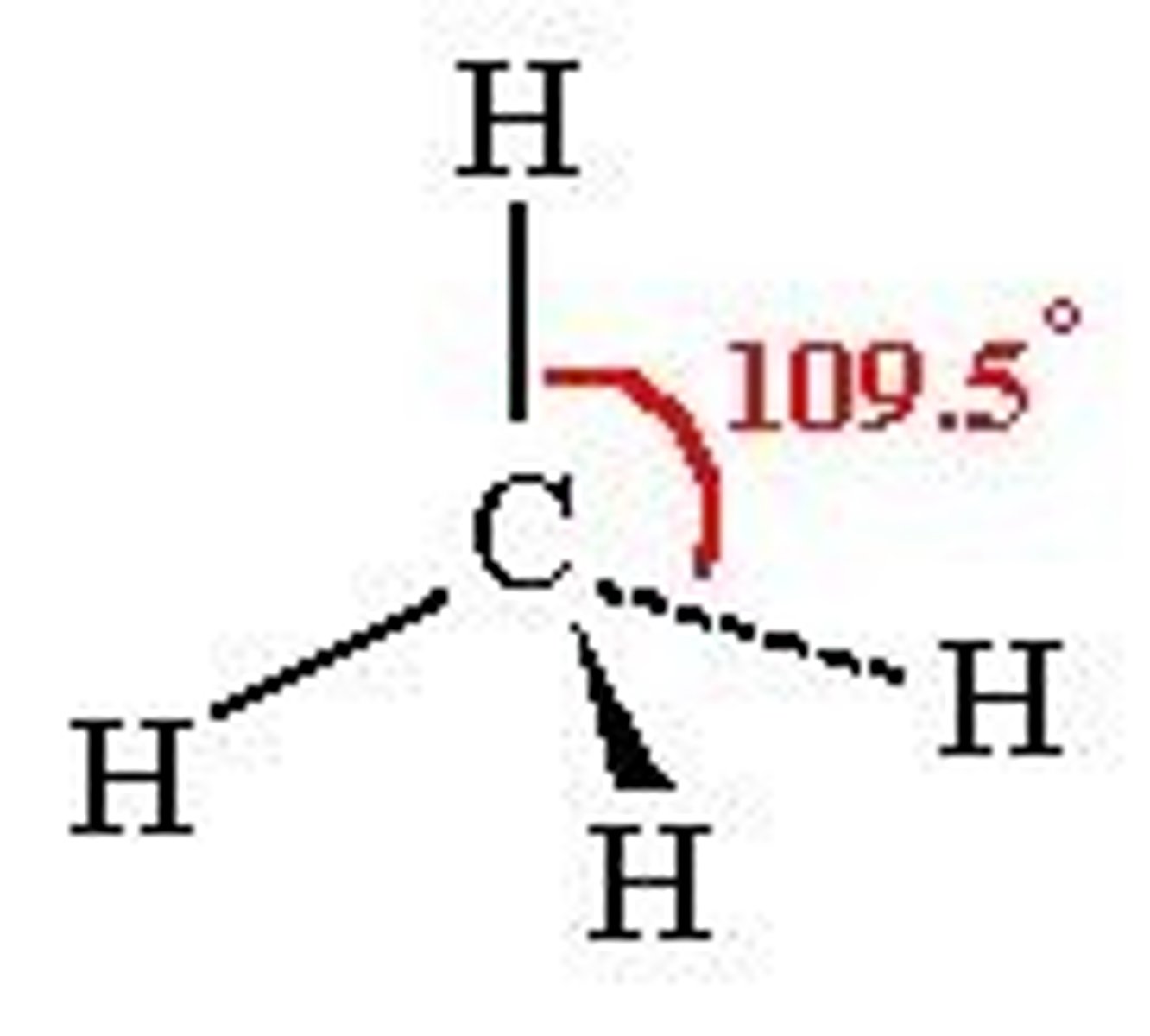
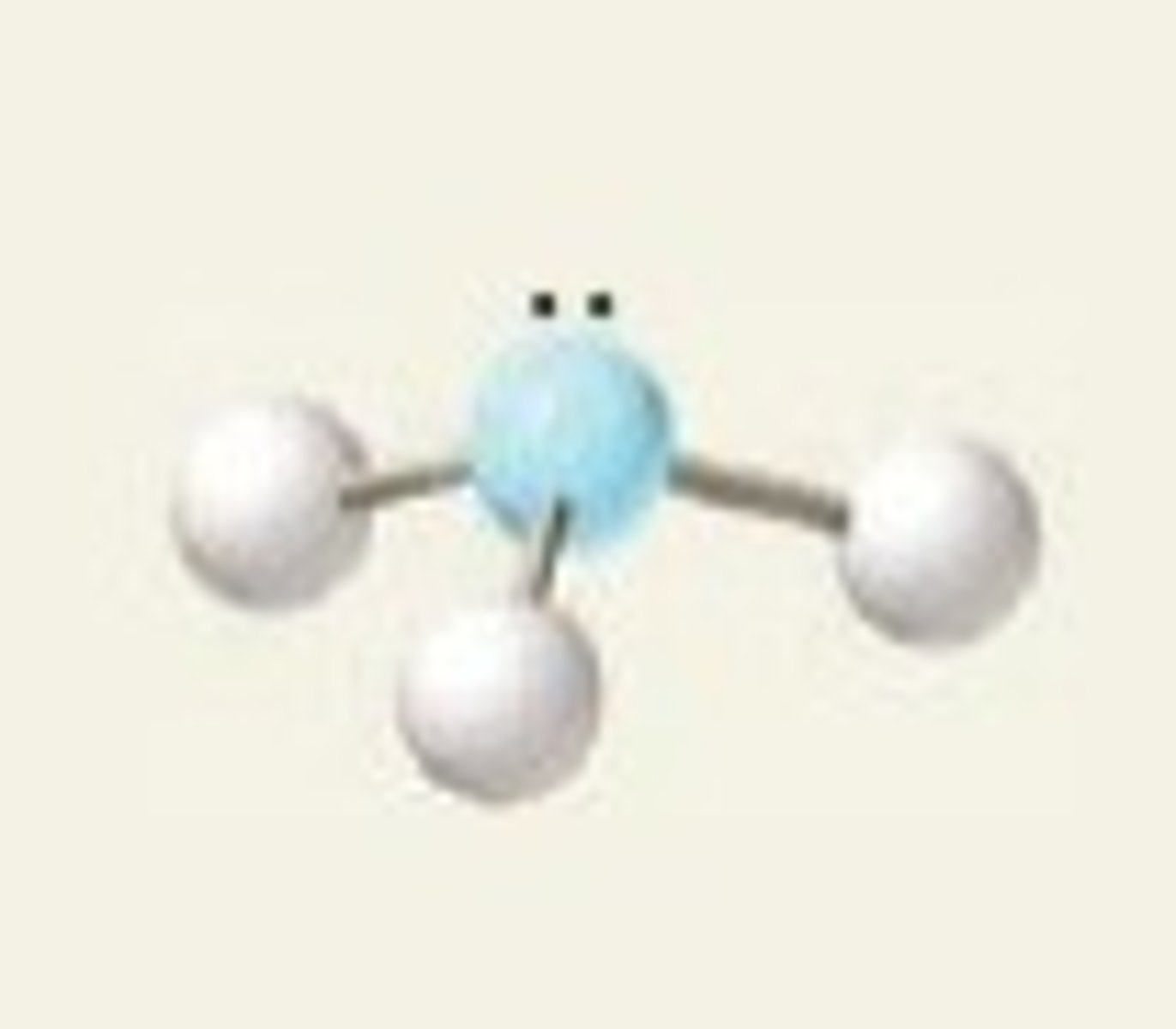
Trigonal Pyrimidal
107
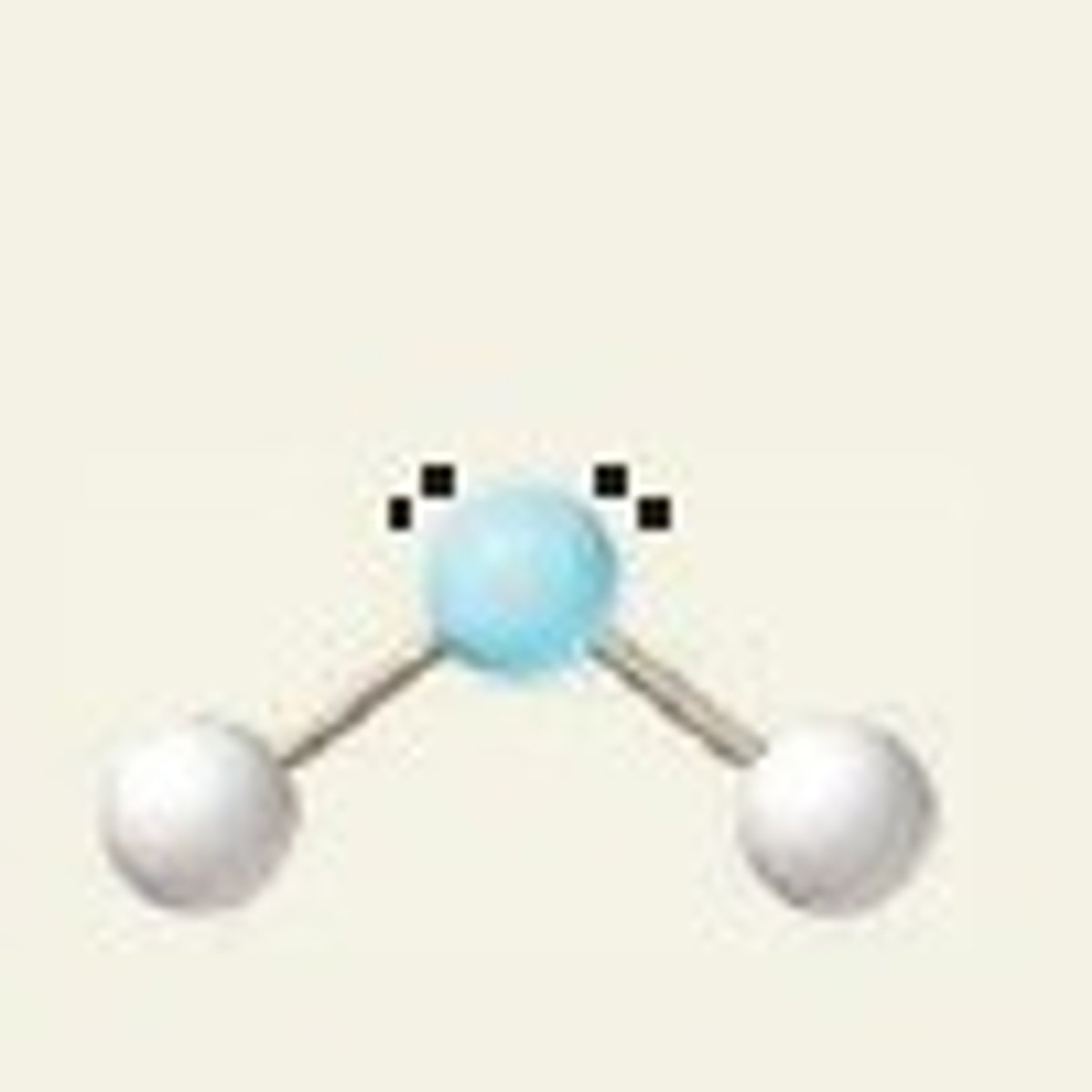
Bent
104.5
What makes a bond polar?
Uneven sharing (0.2-1.7)
What makes a molecule polar?
Uneven distribution
1 lone pair: uneven
What makes a molecule ionic?
0.0-0.2
With an ionic bond you just?
Draw the transfer homie
Sn
TIN YOU FOOL
When potassium forms its most common ion it?
Loses an electron
When sulfur atom forms its most common ion it?
gains two electrons
If the formula for an ionic compound is represented by X2X3 what is the charge of the x ion?
Positive three cause the first one is always positive
Nitrogen and Oxegyn would....
Form a covalent compound
Tetraoxide goes to??
Tetroxide
Decaoxide?
decoxide
When do you add
Di
tri
tetra Etc.
When you are dealing with a covalent bond, which is two nonmetals
when do you draw the lewis dot structure of just the tranfery
when its a ionic bond
When do you use the roman numerals
when dealing with any transition metals
Bond is polar when?
BASIC BINARY IONIC COMPOUNDS
MEyal cation from groups 1,2,or 13 bonded with a nonmetal anion
Ternary ionic-polyatomic ions
MEtal cations and non-metal or polyatomic ions. ALL ABOUT IONS
Binary ionic compounds with transition metals
transition metal cation and non-metal or polyatomic ions
covalent (moleceular compounds)
two or more non-metals (above staircase) use prefixes here, no charges, roman numerals or polyatomic ions