AMINES
1/23
Earn XP
Description and Tags
Preparation Reactions for Amines
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
Preparation of mixture of Amines from Ammonia and Alkyl Halide
(Preparation of R4NX)
R-X + NH3 → R-NH2 (Primary Amine) + H-X (+ R-X) → R-R-NH (Secondary Anime) + H-X (+ R-X) → R-R-R-NH (Tertiary Amine) + H-X (+ R-X) → R-R-R-R-N-X (Quaternary Ammonium Salt)
Reagents for reduction of Nitro Group to form Amines
Sn + HCl
Fe + HCl (gives the most yield)
H2 in the presence of Ni catalyst
These reagents help in removing -NO2 and replace it with -NH2 from an organic compound.
Reagents for reduction of Cyanides to form Amines
H2 in the presence of Ni/Pt/Pd
LiAlH4
Na(Hg) in the presence of an alcohol (R-OH)
Triple bonds of CN break to form one single bond and hydrogen is substituted to each bond, forming CH2-NH2
Reagents for reduction of Isocyanides to form Amines
H2 in the presence of Ni/Pt/Pd
LiAlH4
Triple bonds of CN break to form double bonds and hydrogen is substituted to the bond, forming NH=CH2
Reagents for reduction of Amides to form Amines
LiAlH4
Amides are Ar/R-C=O-NH2
R-C(=O)-NH2 → R-CH2-NH2
Ar-C(=O)-NH2 → Ar-CH2-NH2
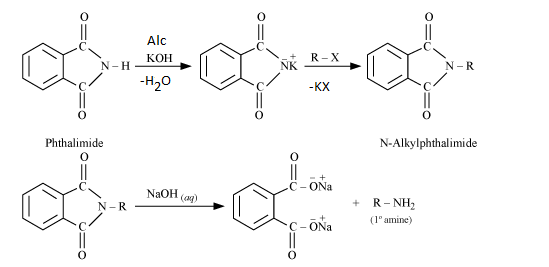
Gabriel Phthalimide Synthesis
Mechanism:
1. Deprotonation of Phthalimide:
Potassium hydroxide (KOH) deprotonates the phthalimide, creating a nucleophilic imide ion. The imide ion is stabilized by resonance due to the presence of two carbonyl groups.
2. Alkylation:
The nucleophilic imide ion attacks the electrophilic carbon of the alkyl halide, displacing the halogen (X) and forming an N-alkyl phthalimide.
3. Hydrolysis:
The N-alkyl phthalimide undergoes base-catalyzed hydrolysis, where the hydroxide ion attacks the carbon-nitrogen bond. This leads to the cleavage of the N-alkyl phthalimide and the formation of the primary amine and a phthalate ion.
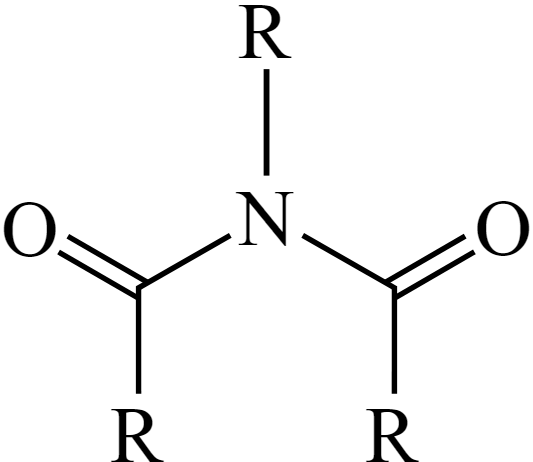
Structure of Imide
1 N attached to 2 (or more) primary or secondary R’s which are doubly bonded with their O’s.
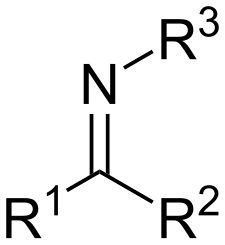
Structure of Imine
1 N (with a lone pair) doubly bonded to a secondary C and singly bonded to an R
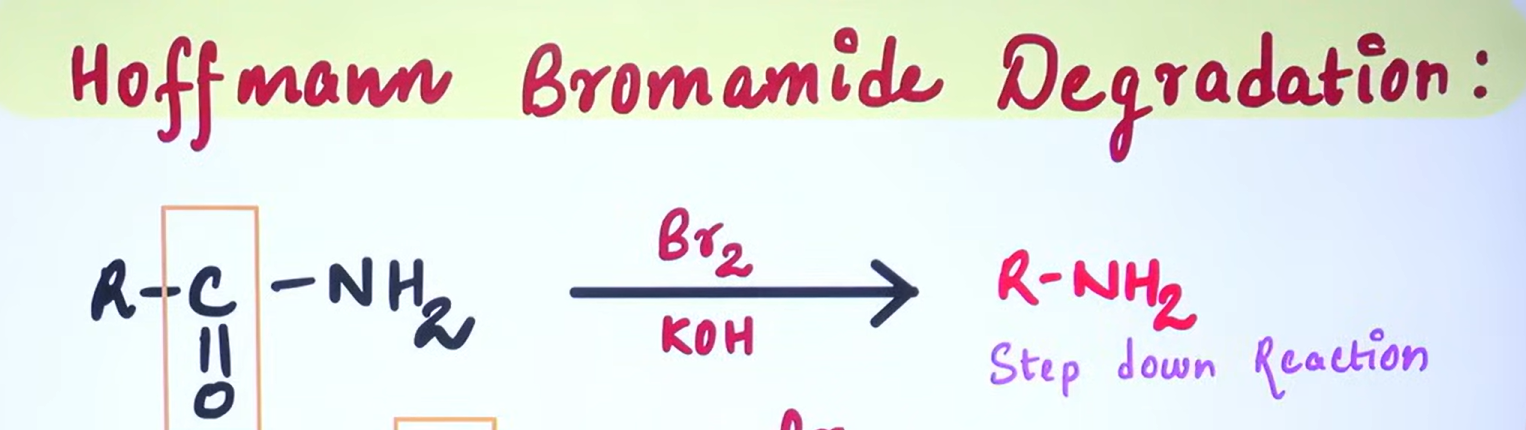
Hoffmann Bromamide Degradation
The Hoffmann Bromamide Degradation is a chemical reaction used to convert primary amides into primary amines with the loss of one carbon atom (Step Down Reaction)
Formation of N-Bromoamide:
The primary amide reacts with bromine in the presence of a strong base, such as sodium hydroxide (NaOH), resulting in the formation of an N-bromoamide.
Deprotonation and Rearrangement:
The strong base deprotonates the N-bromoamide, generating an anion. This anion then undergoes rearrangement, where the alkyl or aryl group attached to the carbonyl carbon migrates to the nitrogen, forming an isocyanate intermediate. During this rearrangement, a bromide ion is expelled.
Hydrolysis of Isocyanate:
The isocyanate intermediate is then hydrolyzed in the presence of water or aqueous base, leading to the formation of a primary amine and the release of carbon dioxide (CO₂).
The overall reaction can be summarized as follows:
RCONH₂ (primary amide) + Br₂ + 4 NaOH → RNH₂ (primary amine) + Na₂CO₃ + 2 NaBr + 2 H₂O
Acylation Reaction of Amines
R-NH2 + R-C=O-Cl → R-NH-C=O-R + HCl
Carbyl Amine Test
R-NH2 + CHCl3 + KOH → R-NC
(Foul smell of isocyanide indicates presence of 1 degree Amine)
Formation of Carbene (CCl2) as an intermediate due to alpha elimination occurs.
Reagents for reactions of Amines with Nitrous Acids (HNO2)
Reagents:
HNO2
NaNO2 + HCl → NaCl + HNO2
Reactions of 1 degree Aliphatic Amines with Nitrous Acids
R-NH2 + HNO2 → R-N2-Cl (Diazonium Salt) → R+ (Carbocation - rearrangement may occur) + H2O → R-OH (Alcohol)
Reactions of 1 degree Aromatic Amines with Nitrous Acids
Ph-NH2 + NaNO2 + HCl → Ph-N2-Cl (Benzene Diazonium Chloride) + H2O → Phenol