Proteoglycans and Glycoproteins
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
Differentiate between proteoglycans and glycoproteins

How do we build complex carbohydrates? List out the nucleotides we use for the different saccharides
Monosaccharides added one at a time
have to be in a nucleotide-activated form.
most frequently = UDP, but can be GDP or CMP.
The nucleotide-activated form is specific for a given monosaccharide.
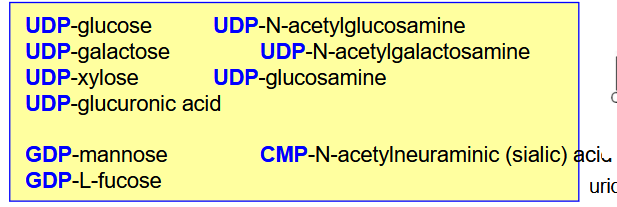
Describe Glycosyltransferases; Function? Why are we using these nucleotides?
catalyzes the addition of monosaccharides to other molecules.
Specific for a given monosaccharide.
hydrolysis of the nucleotide-linkage provides energy for the formation of the glycosidic bond.
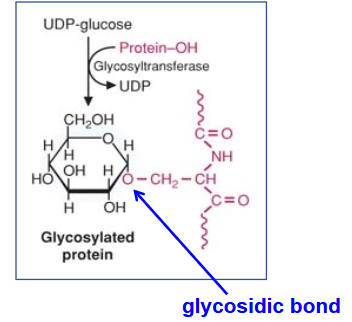
Describe Glycosaminoglycans (GAGs)
(Composition? Attachment? Synthesis? Charge? Function of charge
repeating disaccharide (acidic sugar (glucuronic or iduronic acid) + N-acetylated amino sugar.)
All (but hyaluronic acid) are attached to a protein core
All (but hyaluronic acid) are synthesized in the Golgi. Hyaluronic acid is synthesized at the plasma membrane
All negatively charged.
All (but hyaluronic acid) are sulfated.
negative charges attract water →“sponges”.
They confer resilience to tissues under compressive forces.
Describe Hyaluronic Acid
(Importance? location? Function? Clinical importance?
Widespread structural GAGs in the human body
Main locations: cartilage, synovial fluid, vitreous humor of the eye.
Function:
Major lubricant of the joints.
Essential for embryogenesis, morphogenesis, and wound healing.
Clinical applications:
used in eye surgery (Healon)
treatment of osteoarthritic pain (intraarticular Synvisc injection)
Describe Chondroitin Sulfates
(Sulfation? Location? Function? )
sulfated in the 6th and/or the 4th (*) position of N-acetyl- galactosamine
Largest concentration is in cartilage, (but found in many other tissues.)
Main GAG in aggregating proteoglycans (that aggregate with hyaluronic acid)
What is the most abundant of GAGs in the human body?
Chodroitin Sulfates
Describe Dermatan Sulfate
(Difference? Location? clinical importance?
Differs from chondroitin-4-sulfate:
orientation of carboxyl group
Location:
Mainly found in skin, blood vessels, and heart valves
Clinical importance:
Increased accumulation of dermatan sulfate in mitral valves → thickening and abnormal displacement of the valve into the left atrium of the heart (mitral valve prolapse).
Describe Keratan Sulfate
(exception? types? clinical importance?)
only GAG that does not contain an acidic sugar.
two types of keratan-sulfate (KS):
KS I: linked to the amide of Asn (mainly found in cornea).
KS II: linked to the hydroxyl of Ser (mainly found in cartilage).
Clinical Importance:
Carbohydrate sulfotransferase 6 (CHST6) deficiency →undersulfated KS in cornea → macular corneal dystrophy (cloudy corneas, vision impairment)
differentiate between Heparin and Heparan Sulfate
differ in sulfation pattern (heparin = more sulfated).
Heparan sulfate:
Part of a major proteoglycan (perlecan) in basement membranes
Frequent component of cell surface molecules/receptors
Heparin:
stored in and released by mast cells
Used as anticoagulant in medicine (management of myocardial infarction and deep vein thrombosis)
What are proteoglycans? What type of molecules are they? How are they attached?
Proteins where at least one (GAG) chain is attached.
either cell surface receptors or extracellular matrix molecules.
Attachment:
GAGs are attached to the protein core through a specific linkage region.
either a hydroxyl of Ser or an amide of Asn.
What is aggrecan?
main cartilage proteoglycan (aggrecan) contains ~ 100 GAG chains
mainly chondroitin sulfates with some keratan sulfate.
has a characteristic bottle-brush structure.
How is Aggrecan retained? Function of GAG?
Aggrecan is retained in the cartilage ECM through non- covalent interactions with hyaluronic acid and link protein (a small glycoprotein).
GAG content provides the tissue with resilience under compressive forces
Describe the Homeostasis of Cartilage Proteoglycan
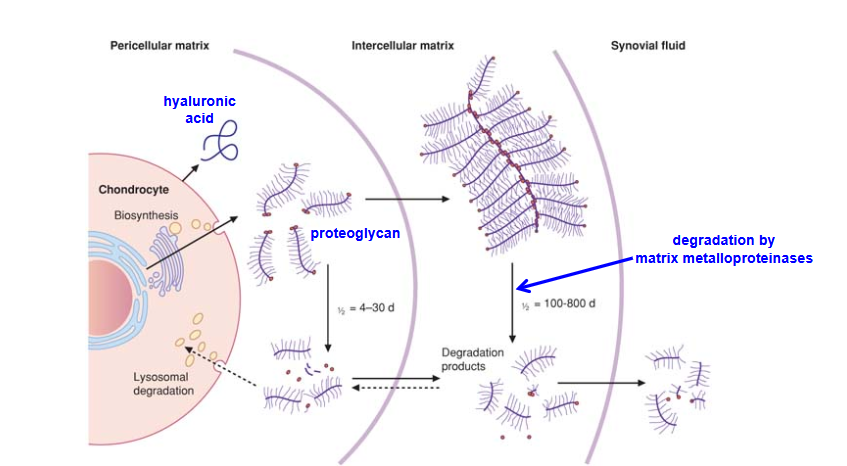
Which diseases are associated with degradation of cartilage?
Osteoarthritis, rheumatoid arthritis, and systemic lupus erythematosus (SLE)
leads to defective cartilage function.
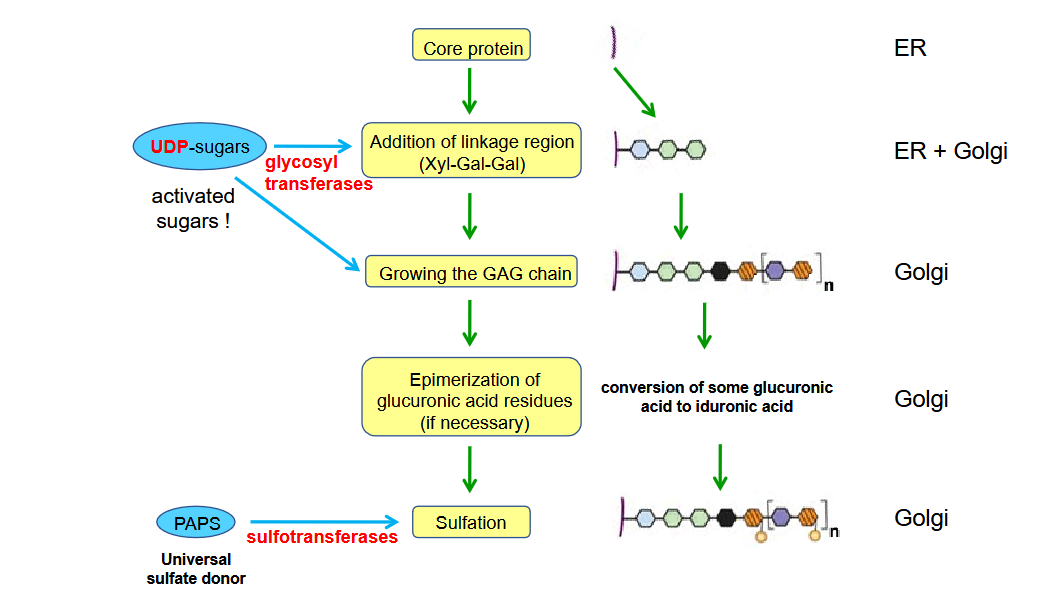
Describe the Synthesis of Proteoglycans
Synthesis of amino sugars (cytosol)
Amino sugars derive from glucose.
Glutamine provides the amino group.
Synthesis of glucuronic acid (cytosol)
From UDP-glucose by oxidation
Synthesis of core protein (endoplasmic reticulum)
Synthesis of the GAG chain (Golgi, but for hyaluronic acid – plasma membrane)
synthesized on trisaccharide links at serine residues and sometimes asparagine residues of the core protein.
Glycosyltransferases produce the growing GAG chain by the addition of the appropriate UDP-sugars.
Sulfation (Golgi)
Sulfotransferases add sulfate groups to the GAG chains.
The sulfate donor is PAPS (3’ phosphoadenosyl-5’-phosphosulfate)
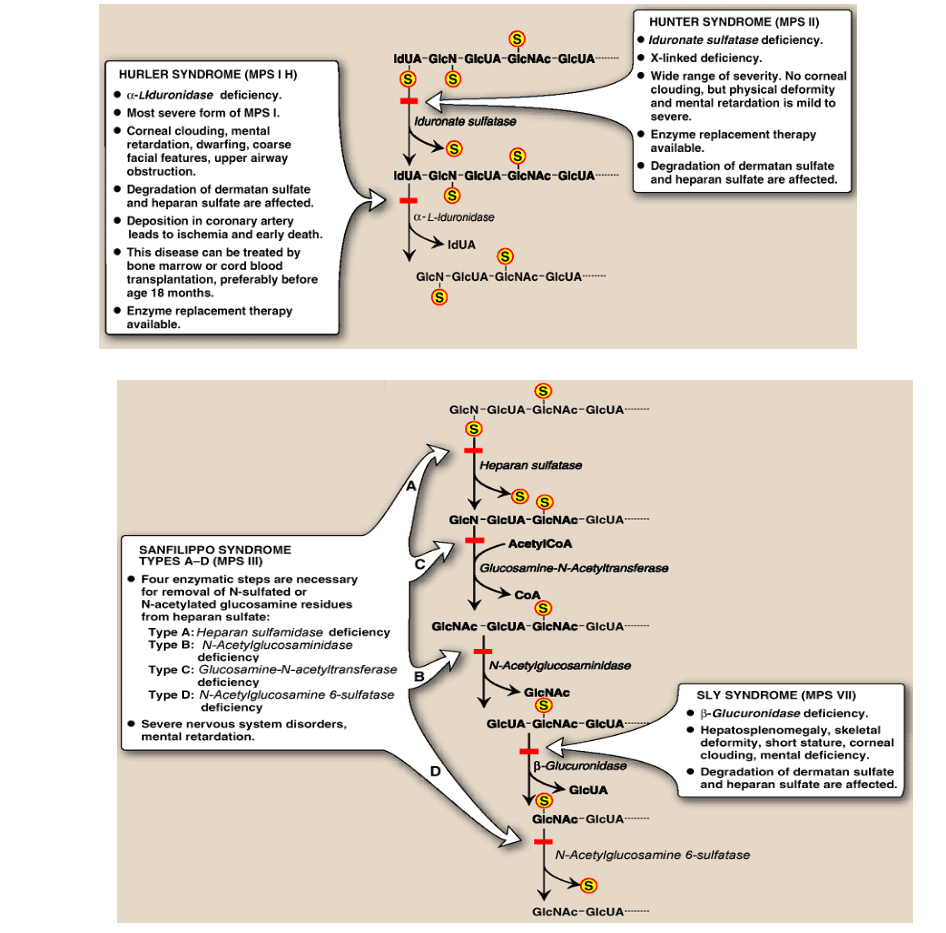
Describe the Degradation of Glycosaminoglycans; Deficiency?
GAGs are taken up by the cell through endocytosis.
GAGs are transported to lysosomes where they are degraded by specific enzymes
Deficient lysosomal degradation of GAGs can cause severe diseases (mucopolysaccharidoses – a group of lysosomal storage diseases)
Draw out the steps of the degradation of Glycosaminoglycans and the relevant diseases/symptoms
Describe glycoproteins? How are they different from proteoglycans? Function?
Proteins with oligosaccharides attached to them covalentl
The oligosaccharides are smaller than GAGs of the proteoglycans.
Function of glycoproteins:
Cell surface recognition molecules/receptors
Includes binding sites for pathogens
Blood group antigens
ECM molecules
laminins, collagens, fibronectin
Mucins
lubricants, surfactants
Plasma proteins
oligosaccharide chains increase the solubility of the protein
What are the two types of Oligosaccarides? Compare/Contrast them
O-linked
attached to the hydroxyl group of Ser or Thr.
linear structure
N-linked
attached to the amide group of asparagine.
branched structure
can be either mannose-rich or complex.
Describe the synthesis of Glycoprotein
protein synthesized in ER
Monosaccharides have to be in nucleotide activated form to be added (usually UDP-linked)
Monosaccharides are added by glycosyltransferases
Differentiate between synthesis of O-linked glycoproteins and N-linked glycoproteins
O-linked glycoproteins:
chain built in Golgi.
N-linked glycoproteins:
chain starts building on lipid (dolichol) in the membrane of ER
(Dolichol is a side- product of cholesterol synthesis)
oligosaccharide precursor transferred to the protein in the ER.
Oligosaccharide synthesis is completed in the Golgi.
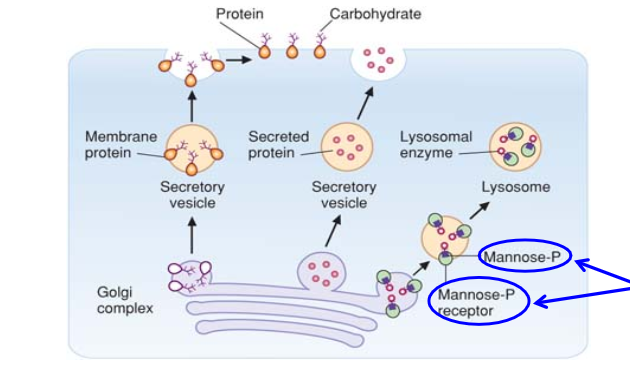
Describe the Role of N-Linked Oligosaccharides in Lysosomal Targeting of Proteins
Delivering enzymes into lysosomes requires the
phosphorylation of a mannose on an N-linked oligosaccharide of the lysosomal enzyme.
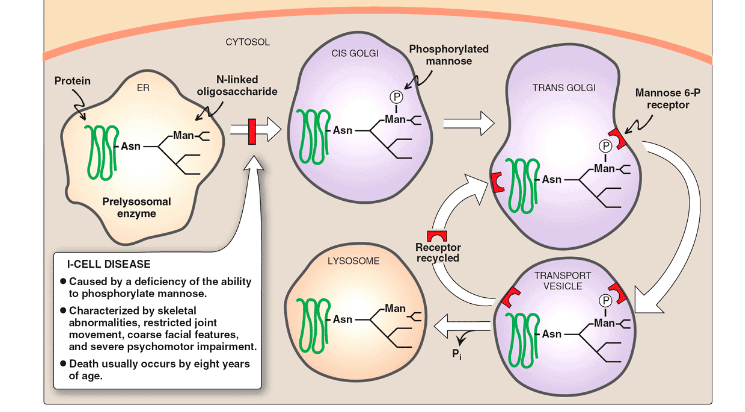
Describe Mucolipidosis II (I-Cell Disease); Symptoms?
Deficient mannose phosphorylation on lysosomal enzymes
Lysosomal enzymes are not delivered to the lysosomes (instead they are secreted).
Macromolecules are not degraded (enlarged lysosomes, Inclusion bodies)
What does Osteonectin do?
Bone glycoprotein
Interacts with bone minerals and collagen fibers.
Facilitates collagen fiber mineralization.
What does link protein do?
Cartilage glycoprotein
Stabilizes the interaction between hyaluronan and aggrecan.
What does Dystroglycan do
Skeletal muscle cell membrane glycoprotein
Binds to laminins and mediate cell attachment to the adjacent basement membrane.
Defective glycosylation of dystroglycan leads to muscular dystrophy