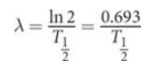MCAT Physics and Math - Atomic and Nuclear Phenomena
1/42
Earn XP
Description and Tags
535
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
43 Terms
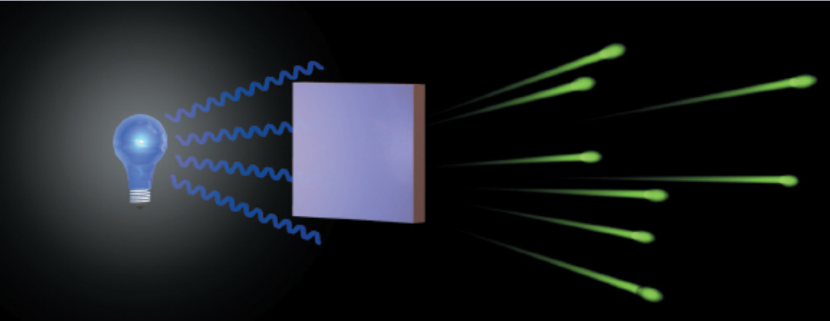
photoelectric effect
When light of a sufficiently high frequency (typically, blue to ultraviolet light) is incident on a metal in a vacuum, the metal atoms emit electrons; light beams of greater intensity produce proportionally larger current; “all-or-nothing” response
current
net charge flow per unit time
threshold frequency (fT)
minimum frequency of light that causes ejection of electrons; depends on the type of metal
if the frequency of the incident photon is less (f < fT) → no electron will be ejected (insufficient energy)
if the frequency of the incident photon is more (f < fT) → electron will be ejected (sufficient energy)
maximum kinetic energy of the ejected electron
equal to the difference between hf and hfT; exact kinetic energy because the actual energy can be anywhere between 0 and Kmax
Kmax = hf − W = h(f - fT)
photons
light beam consists of an integral number of light quanta
energy of photon
proportional to the frequency of the light
E = hf
where E is the energy of the photon of light, h is Planck’s constant, and f is the frequency of the light
Planck’s constant (h)
6.626 ×10−34 J·s
work function
minimum energy required to eject an electron; related to the threshold frequency
W = hfT
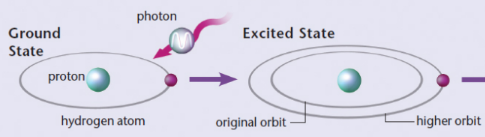
atomic absorption
electron can jump from a lower-energy to a higher-energy orbit by absorbing a photon of light of precisely the right frequency to match the energy difference between the orbits
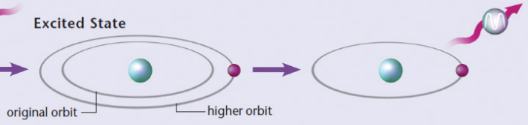
atomic emission
an electron falls from a higher-energy level to a lower-energy level, a photon of light is emitted with an energy equal to the energy difference between the two orbits
infrared (IR) spectroscopy
determine chemical structure; different bonds will absorb different wavelengths of light
UV–Vis spectroscopy
the absorption of light by bonds in the visible and ultraviolet range
Absorption spectra
represented as a color bar with peak areas of absorption represented by black lines OR as a graph with the absolute absorption as a function of wavelength
phenolphthalein
indicator
acidic - transparent = absorb no visible light
basic - bright pink = absorbing all but the longer wavelengths of visible light
fluorescence
After being excited to a higher energy state by ultraviolet radiation, the electron in the fluorescent substance returns to its original state in two or more steps; at each step, a photon is emitted with a lower frequency (longer wavelength) than the absorbed ultraviolet photon
mass defect
difference between the sum of the masses of all of the protons and neutrons within it and the actual mass of every nucleus; result of matter that has been converted to energy
equivalence of matter and energy
E = mc2
where E is energy, m is mass, and c is the speed of light
1 g of mass = 89.9 TJ = 21.5 billion Kcal
nucleons
protons and neutrons, attracted by strong nuclear force
strong nuclear force
strong enough to more than compensate for the repulsive electromagnetic force between the protons; only acts over extremely short distances (less than a few times the diameter of a proton or neutron)
binding energy
bonded nucleons are at a lower energy level than the unbonded constituents; this difference in energy must be radiated away in the form of heat, light, or other electromagnetic radiation before the mass defect becomes apparent
weak nuclear force
contributes to the stability of the nucleus, but is about one-millionth as strong as the strong nuclear force
four fundamental forces of nature
strong nuclear force
weak nuclear force
electrostatic forces
gravitation
isotopic notation
elements are preceded by their atomic number as a subscript and mass number as a superscript
AZX
atomic number (Z)
the number of protons in the nucleus
mass number (A)
the number of protons plus neutrons
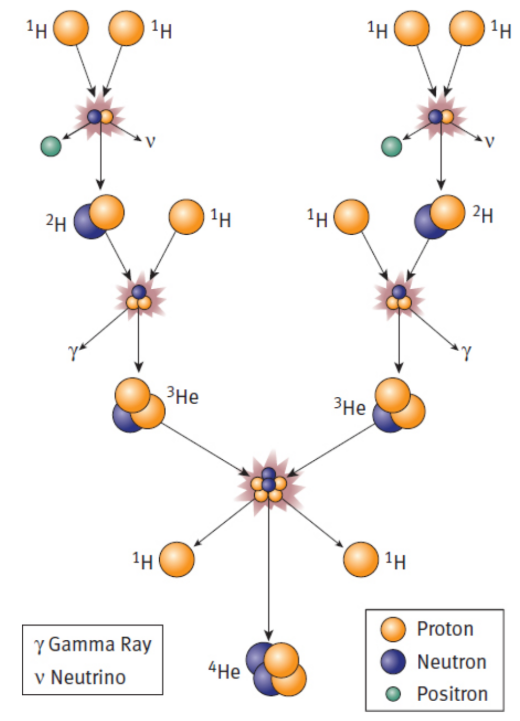
Fusion
a process by which small nuclei combine to form a larger nucleus
ex. stars (hydrogen → helium); power plants
Fission
a process by which a large nucleus splits into smaller nuclei; rarely spontaneous; often requires the absorption of a low-energy neutron
Radioactive decay
naturally occurring spontaneous decay of certain nuclei accompanied by the emission of specific particles
parent nucleus (X)
undergoes nuclear decay
daughter nucleus (Y)
formed from nuclear decay
Isotope Decay Arithmetic
AZX → A’Z’Y + emitted particle
the sum of the atomic numbers and the mass numbers must be the same on both sides
Alpha decay
emission of an α-particle
AZX → A-4Z-2Y + 42α
α-particle
two protons, two neutrons, and zero electrons; massive; twice the charge as β-particle; interact with matter; low penetrating power
42He
Beta decay
a neutron decays into a proton, β--particle, and an antineutrino (ν)
AZX → AZ+1Y + β−
β-particle
an electron; very light; more penetrating than alpha radiation
e- or β-
positron emission
proton is converted into a neutron, a β+-particle, and a neutrino
AZX → AZ-1Y + β+
positron
mass of an electron but carries a positive charge
e+ or β+
Gamma decay
emission of γ-rays without changing the mass number or the atomic number; high-energy state of the parent nucleus may be represented by an asterisk
AZX* → AZX + γ
γ-rays
high-energy (high-frequency) photons; no charge; lower the energy of the parent nucleus
Electron capture
rare process; reverse of β− decay
AZY + e− → AZ-1Y
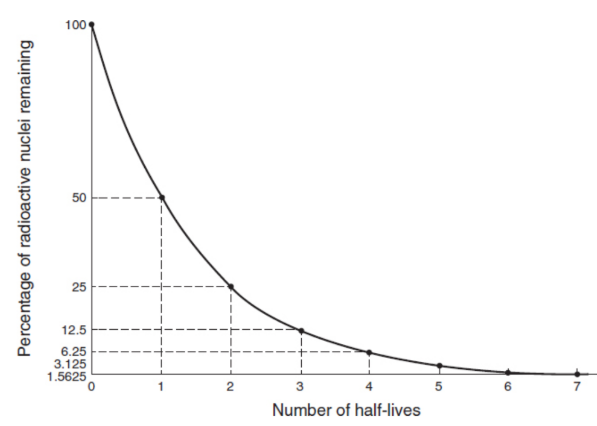
half-life (T1/2)
time it takes for half of the sample to decay; asymptotically approaches zero
exponential decay
the rate at which the nuclei decay is proportional to the number that remain
n = n0e−λt
where n0 is the number of undecayed nuclei at time t = 0
decay constant & half-life