RA 9288
1/65
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
66 Terms
Newborn Screening Act of 2004
RA 9288
Newborn Screening (NBS)
a simple procedure to find out if a baby has a congenital disorder that may lead to mental retardation or even death if left untreated
Newborn Screening (NBS) Importance
Most babies with metabolic disorders look “normal” at birth. By doing NBS, metabolic disorders may be detected even before clinical signs and symptoms are present. As a result of this, treatment can be given early to prevent consequences of untreated conditions.
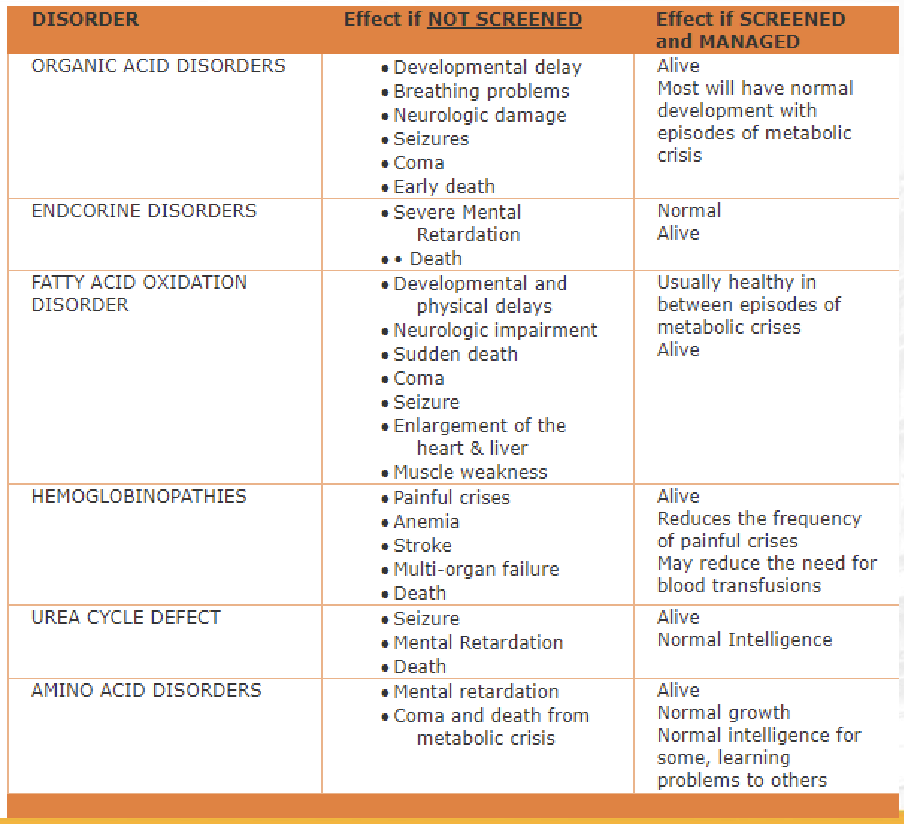
Screening Disorders
Comprehensive Newborn Screening (NBS) Program
was integrated as part of the country’s public health delivery system with the enactment of the Republic Act no. 9288 otherwise known as Newborn Screening Act of 2004
Department of Health (DOH)
acts as the lead agency in the implementation of the law and collaborates with other National Government Agencies (NGA) and key stakeholders to ensure early detection and management of several congenital metabolic disorders.
1. congenital hypothyroidism (CH)
2. congenital adrenal hyperplasia (CAH)
3. phenylketonuria (PKU)
4. glucose-6-phosphate dehydrogenase (G6PD) deficiency
5. galactosemia (GAL)
6. maple syrup urine disease (MSUD)
Newborn screening program in the Philippines currently includes screening of six disorders:
National Policy and Strategic Framework on Expanded Newborn Screening for 2017-2030
Administrative Order No. 2018-0025
Guidelines on the Implementation of the Expanded Newborn Screening Program
Administrative Order No. 2014-0045
▪ hemoglobinopathies and
▪ additional metabolic disorders, namely, organic acid, fatty acid oxidation, and amino acid disorders
The expanded screening will include 22 more disorders:
Newborn Screening Centers (NSC)
The RA 9288 defines the establishment and accreditation of __
1. Newborn Screening Center - NIH at the University of the Philippines Manila
2. Newborn Screening Center - Visayas located at West Visayas State University Medical Center, Iloilo City
3. Newborn Screening Center - Mindanao located at the Southern Philippines Medical Center, Davao City
4. Newborn Screening Center - Central Luzon in Angeles City, Pampanga
5. Newborn Screening Center - Southern Luzon in Tanauan City, Batangas
6. Newborn Screening Center - Northern Luzon in Batac City, Ilocos Norte
six operational NSCs in the country
ideally done immediately after 24 hours from birth
When is it done?
A few drops of blood are taken from the baby’s heel, blotted on a special absorbent filter card and then sent to Newborn Screening Center (NSC)
How is it done?
physician, nurse, medical technologist or trained midwife.
Who will collect the sample?
“Declaring the 1st Week of October of Each Year as “National Newborn Screening Week”
Proclamation No. 540
January 20, 2004
Proclamation No. 540 dated
19
RA 9288 sections
President Gloria Macapagal-Arroyo
RA 9288 approved by
April 7, 2004
RA 9288 date
Title
Section 1
Newborn Screening Act of 2004
Section 1 – Title
Declaration of Policy
Section 2
Section 2 – Declaration of Policy
▪ In pursuit of such policy, the State shall institutionalize a national newborn screening system that is comprehensive, integrative and sustainable, and will facilitate collaboration among government and non-government agencies at the national and local levels, the private sector, families and communities, professional health organizations, academic institutions, and non-governmental organizations.
▪ The National Newborn Screening System shall ensure that every baby born in the Philippines is offered the opportunity to undergo newborn screening and thus be spared from heritable conditions that can lead to mental retardation and death if undetected and untreated.
Objectives
Section 3
Section 3 - Objectives
1. To ensure that every newborn has access to newborn screening for certain heritable conditions that can result in mental retardation, serious health complications or death if left undetected and untreated;
2. To establish and integrate a sustainable newborn screening system within the public health delivery system;
Section 3 - Objectives
3. To ensure that all health practitioners are aware of the advantages of newborn screening and of their respective responsibilities in offering newborns the opportunity to undergo newborn screening; and
4. To ensure that parents recognize their responsibility in promoting their child's right to health and full development, within the context of responsible parenthood, by protecting their child from preventable causes of disability and death through newborn screening.
Definition
Section 4
Obligation to Inform
Section 5
Section 5 – Obligation to Inform
Any health practitioner who delivers, or assists in the delivery, of a newborn in the Philippines shall, prior to delivery, inform the parents or legal guardian of the newborn of the availability, nature and benefits of newborn screening.
Performance of Newborn Screening
Section 6
twenty-four (24) hours; three (3) days
Section 6 - shall be performed after __ of life but not later than __ from complete delivery of the newborn
intensive care; seven (7) days of age
Section 6 - newborn that must be placed in __ in order to ensure survival may be exempted from the 3-day requirement but must be tested by __
parent(s); practitioner
Section 6 - joint responsibility of the __ and the __ or other person delivering the newborn to ensure that newborn screening is performed
Refusal to be Tested
Section 7
Section 7 – Refusal to be Tested
A parent or legal guardian may refuse testing on the grounds of religious beliefs, but shall acknowledge in writing their understanding that refusal for testing places their newborn at risk for undiagnosed heritable conditions
Continuing Education, Re-education and Training Health Personnel
Section 8
Section 8 – Continuing Education, Re-education and Training Health Personnel
The DOH, with the assistance of the NIH and other government agencies, professional societies and non- government organizations, shall:
1. conduct continuing information, education, re-education and training programs for health personnel on the rationale, benefits, procedures of newborn screening; and
2. disseminate information materials on newborn screening at least annually to all health personnel involved in material and pediatric care
Licensing and Accreditation
Section 9
Section 9 – Licensing and Accreditation
The DOH and the Philippine Health Insurance Corporation shall require health institutions to provide newborn screening services as a condition for licensure or accreditation
Lead Agency
Section 10
Section 10 – Lead Agency
The DOH shall be the lead agency in implementing this Act. For purposes of achieving the objectives of this Act, the DOH shall:
1. Establish the Advisory Committee on Newborn Screening:
2. Develop the implementing rules and regulations for the immediate implementation of a nationwide newborn screening program within one hundred eight (180) days from the enactment of this Act;
Section 10 – Lead Agency
3. Coordinate with the Department of the Interior and Local Government (DILG) for implementation of the newborn screening program;
4. Coordinate with the NIH Newborn Screening Reference Center for the accreditation of Newborn Screening Centers and preparation of defined testing protocols and quality assurance programs.
Advisory Committee on Newborn Screening
Section 11
Section 11 – Advisory Committee on Newborn Screening
To ensure sustained inter-agency collaboration, the Advisory Committee on Newborn Screening is hereby created and made an integral part of the Office of the Secretary of the DOH
• review annually and recommend conditions to be included in the newborn screening panel of disorders;
• review and recommend the newborn screening fee to be charged by Newborn Screening Centers;
Section 11 – Advisory Committee on Newborn Screening
• review the report of the Newborn Screening Reference Center on the quality assurance of the National Screening Centers and
• recommend corrective measures as deemed necessary
o Secretary of Health - Chairman
o Executive Director of the NIH - Vice Chairperson
o Undersecretary of the DILG
o Executive Director of the Council for the Welfare of Children
o Director of the Newborn Screening Reference Center
o three (3) representatives appointed by the Secretary of Health who shall be a pediatrician, obstetrician, endocrinologist, family physician, nurse or midwife, from either the public or private sector
Section 11 – Advisory Committee on Newborn Screening - 8 members of committee
Section 11 – Advisory Committee on Newborn Screening - 8 members of committee
▪ The three (3) representatives shall be appointed for a term of three (3) years, subject to their being reappointed for additional three (3) years period for each extension.
▪ The Committee shall meet at least twice a year.
▪ The NIH shall serve as the Secretariat of the Committee.
Establishment and Accreditation of Newborn Screening Centers
Section 12
Section 12 – Establishment and Accreditation of Newborn Screening Centers
The DOH shall ensure that Newborn Screening Centers are strategically located in order to be accessible to the relevant public and provide services that comply with the standards approved by the Committee upon the recommendation of the NIH.
Section 12 – Establishment and Accreditation of Newborn Screening Centers
Newborn Screening Center shall: 1. have a certified laboratory performing all tests included in the newborn screening program,
2. have a recall/follow up programs for infants found positive for any and all of the heritable conditions;
Section 12 – Establishment and Accreditation of Newborn Screening Centers
Newborn Screening Center shall:
3. be supervised and staffed by trained personnel who have been duly qualified by the NIH; and
4. submit to periodic announced or unannounced inspections by the Reference Center in order to evaluate and ensure quality Newborn Screening Center performance.
Section 13 – Establishment of a Newborn Screening Reference Center
The NIH shall establish a Newborn Screening Reference Center, which shall be responsible for the national testing database and case registries, training, technical assistance and continuing education for laboratory staff in all Newborn Screening Centers.
Establishment of a Newborn Screening Reference Center
Section 13
Quality Assurance
Section 14
Section 14 – Quality Assurance
▪ The NIH Newborn Screening Reference Center shall be responsible for:
drafting and ensuring good laboratory practice standards for newborn screening centers,
establishing an external laboratory proficiency testing and certification program.
• act as the principal repository of technical information relating to newborn screening standards and practices,
• provide technical assistance to newborn screening centers
Database
Section 15
Section 15 – Database
▪ NIH Newborn Screening Reference Center shall maintain a national database of patients tested and a registry for each condition
▪ submit reports annually to the Committee and to the DOH on the status of and relevant health information derived from the database
▪ plan for long-term outcome evaluation of newborn screening
Newborn Screening Fees
Section 16
Section 16 – Newborn Screening Fees
▪ the newborn screening fee shall be divided and set aside for the following purposes
o at least four percent (4%) to the DOH's Centers for Health Development or its future equivalent to be spent solely for follow-up services, education and other activities directly related to the provision of newborn screening services
Section 16 – Newborn Screening Fees
o at least four percent (4%) to the Newborn Screening Centers for human resource development and equipment maintenance and upgrading
o at least four percent (4%) to the NIH Newborn Screening Reference Center for overall supervision, training and continuing education, maintenance of national database, quality assurance program and monitoring of the national program
o and the balance for the operational and other expenses of the Newborn Screening Center
Repealing Clause
Section 17
Separability
Section 18
Effectivity
Section 19