6. Neisseria meningitidis, Streptococcal Meningitis, Toxoplasmosis and Lyme disease
1/58
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
59 Terms
meningitis
inflammation of the menges
(dura, arachnoid, pia)
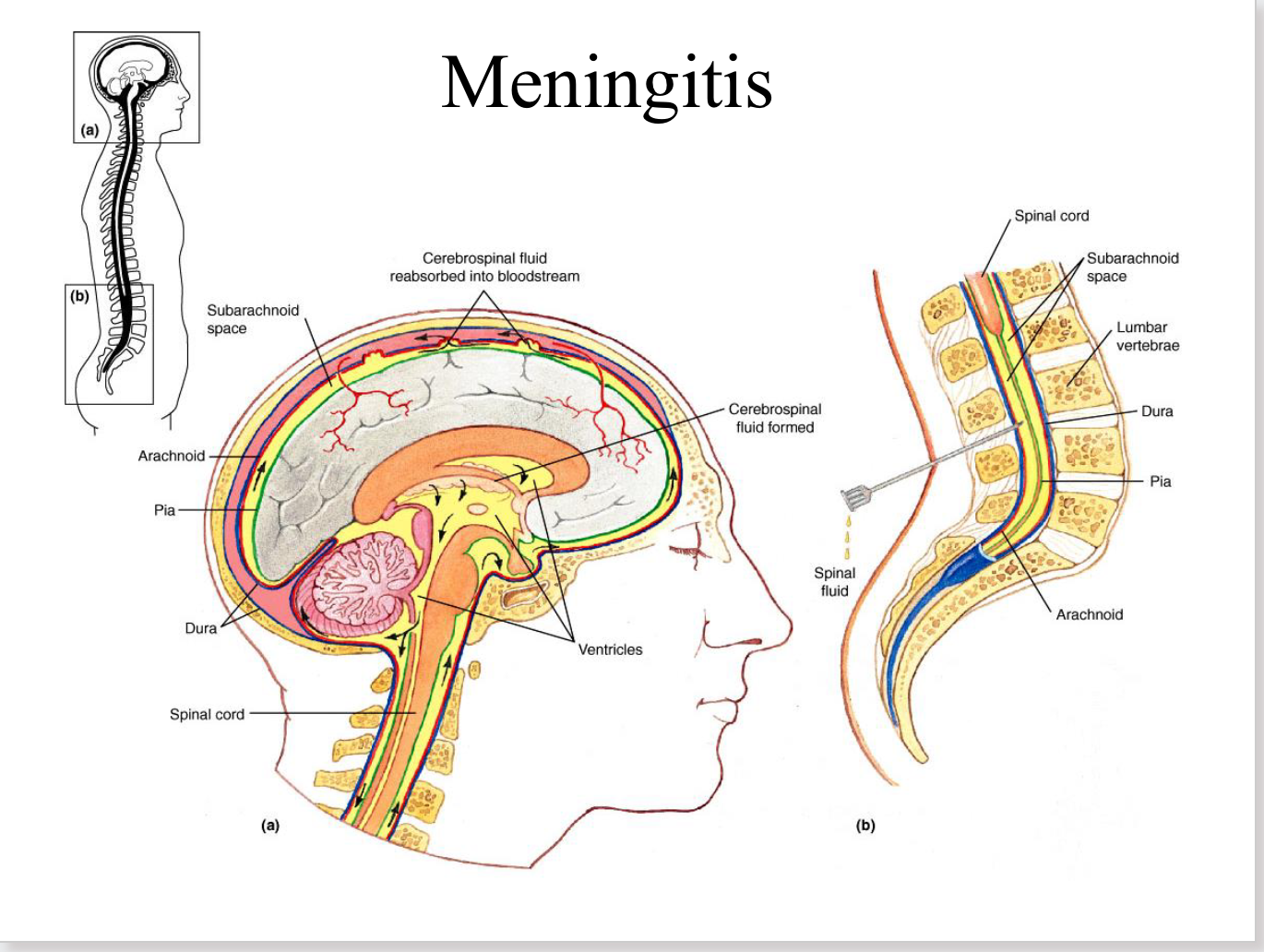
effects of meningitis
10-15% fatality
20% of recovered patients have permanent damage:
– neurological damage
– hearing loss
– defects in spatial learning
Presence of _____ is a common feature to almost all meningitis-causing bacteria
capsule
Many meningitis-causing bacteria are members of
the normal microbiota
– N. meningitidis, H. influenza, S. pneumoniae, E. coli
Neisseria meningitidis
2nd most common cause of community- acquired meningitis in adults
Commonly found in nasopharynx of heathy people
Gram-negative diplococci (Meningococcus)
Covered w/ polysaccharide capsule
capsule
Protects from antibody-mediated phagocytosis
if antibody is directed against a surface protein
Protects from complement-mediated killing
also basis for vaccine-induced antibody recognition
Meningococcal Meningitis
Caused by Neisseria meningitidis
Occurs primarily in young children-teenagers
Can progress very rapidly; life-threatening
Untreated: ~100% fatal
~10% fatal when treated with antibiotics
Symptoms of meningococcal meingitis
Mild cold, sudden onset of severe headache, fever, neck and back pain, nausea, vomiting
“petechiae” may occur in skin from blood vessel hemorrhages
Can develop shock and die within 24 hrs due to release of endotoxin
cell wall LPS of Gram- bacteria (LOS for Neisseria)
Usually slower, with time for treatment
Low incidence of long-term effects (deafness, arthritis)
Meningococcal Pathogenesis
Infection by airborne droplets: person-person
Upper respiratory tract
incubation period 1-7 days
Attach to host cells via pili
Invade respiratory epithelium + gain access to bloodstream → release of endotoxin can lead to shock
Travel from blood to meninges and spinal fluid → crosses blood-brain barrier; grows quickly
Immune cell infiltration → Inflammation of meninges → death of tissue and increases pressure in the brain
Blood-Brain Barrier
characterized by tight intercellular adhesion junctions to prevent crossing of
the barrier
N. meningitidis Crossing the BBB
After attachment, microcolonies of N. meningitidis trigger cell signaling in endothelial cells
the reorganization of proteins maintaining the tight junctions to the area under the microcolony.
This loosens the tight junction and allows paracellular passage between cells of endothelial wall
Some evidence for transcytosis/invasion through endothelial cells as well
N. meningitidis virulence factors
Pili
Porin proteins
Opa proteins
Release membrane blebs containing LOS
IgA protease
Pili
mediate attachment to Attach to non-ciliated columnar epithelium of nasopharynx and brain microvascular endothelial cells (BMECs)
Porin proteins
facilitate attachment to cells
Opa proteins
facilitate attachment and confer resistance to serum proteins
Release membrane blebs containing LOS
shorter form called LOS not LPS (lipo-oligosaccharide, LOS)
– decoy for antibodies to Neisseria?
IgA protease
cleaves IgA antibodies to inactive them
some strains make beta-lactamases
Meningococcal Diagnosis
Gram stain: CSF (meningitis) or blood (septicemia)
Culture is also possible, but difficult
Immunity to Meningococcus
Antibodies are important for protection
Neonates are protected for 6 months by maternal antibodies
Complement is important for clearing infections
LOS can trigger inflammation leading to vascular damage, petechiae
Epidemiology and Vaccine
N. meningitidis is more likely to cause large outbreaks of
meningitis than Haemophilus influenzae or Streptococcus
pneumonia
Most N. meningitidis infections are restricted to nose and throat, then cleared
5-15% healthy people carry organism, transient carriers
Can be spread by asymptomatic carriers
Vaccine given in teen years and/or where an outbreak is occurring
Short term immunity, not effective on children <2yr
Often give prophylactic antibiotics during an outbreak
New vaccine approved in 2005, recommended for high school and
college age adolescents, MenACWY
Protects against 4 different capsule antigens (serotypes)
MenB vaccine introduced 2015
N. meningitidis treatment
Antibiotic of choice: ceftriaxone, first choice
can switch to penicillin if susceptible
Does not clear N. meningitidis from asymptomatic carriers
Alternatives: chloramphenicol
Chemoprophylaxis of exposed pt contacts
July 2016: Meningitis in Camp Councilor at Lifetime Fitness Gym Daycare
July 5-11, 2016 up to 213 children and 39 adult employees exposed to infected councilor
Those potentially exposed, recommended to be assessed by Dr. and put on prophylactic antibiotics
Councilor was 21-year old Central Michigan University student
Councilor dies of meningococcal meningitis on July 14
Meningococcemia signs + symptoms
Septicemia
Thrombosis of small blood vessels
Small petechial skin lesions on trunk & lower limbs
Can coalesce to form hemorrhagic bullae
Shock, disseminated intravascular coagulation (DIC)
Adrenal gland destruction: Waterhouse-Friderichsen syndrome
Starts with mild disease symptoms: low-grade fever, arthritis, petechial rash
Often fatal
Responds well to antibiotics
Septicemia
blood infection
not necessarily spread to brain
Group B Streptococci
Streptococcus agalactiae
Significant cause of septicemia, pneumonia, and meningitis in neonates
~10,000-15,000 episodes of invasive GBS disease each year in newborns in US-prior to preventive more recent measures
now more like 1500/year
Can also cause skin and soft-tissue infections, leading to sepsis
Guidelines to Prevent GBS Disease in Newborns
All pregnant women screened at 35-37 wks gestation for anogenital GBS infection
Antibiotics (penicillin) should be administered i.v. during labor (intrapartum) if:
– mother is a GBS carrier
– less than 37 weeks gestation
– membrane ruptures >18 hrs prior to delivery
– temperature of 100.4o F (38.0o C)
– previous delivery of infant with GBS disease
Late Onset Case of GBS Bacteremia in a Newborn (2016, Oregon)
Mom at 37 wks gestation was GBS-negative
Shortly afterbirth, infant developed respiratory distress and transferred to NICU
Blood culture + for GBS, CSF negative
Treated for 11 days with ampicillin
5-days later, re-admitted, again colonized with same strain of GBS
CSF still sterile, expressed breast milk did not contain GBS
What is source of infection?
Mom had been eating capsules derived from her placental tissue that had
been prepared by a commercial vendor
Vendor protocol is to clean and then dehydrate material with heat (46-71˚C) prior to making capsules which are then stored at room temperature
No standards exist for proper processing of placenta for consumption
Capsules were found to be contaminated with viable GBS genetically identical to re-infecting strain
Mom instructed to stop eating capsules and infant treated successfully for 14 days with antibiotics (ampicillin and gentamicin)
Streptococcus pneumoniae
Normal microbiota of nasopharynx
Important opportunistic pathogen
pneumonia, meningitis, sinusitis, otitis media (ear infection)
most common cause of bacterial meningitis in adults
Streptococcus pneumoniae
how does s. pneumoniae cause meningitis
Can damage BBB cells with a toxin called pneumolysin or facilitate invasion of BBB endothelial cells with LTA (Gram+ specific)
Hallmark on gram straining or CSF examination for s. pneumoniae meningitis
Gram + Diplococci
More in respiratory module
Toxoplasmosis
Caused by Toxoplasma gondii passed in feces
Cats infected by predation
Stable in soil/water for months
Either indirect through intermediate host or direct via food/water
Vertical transmission during pregnancy
incidence of Toxoplasma Infections
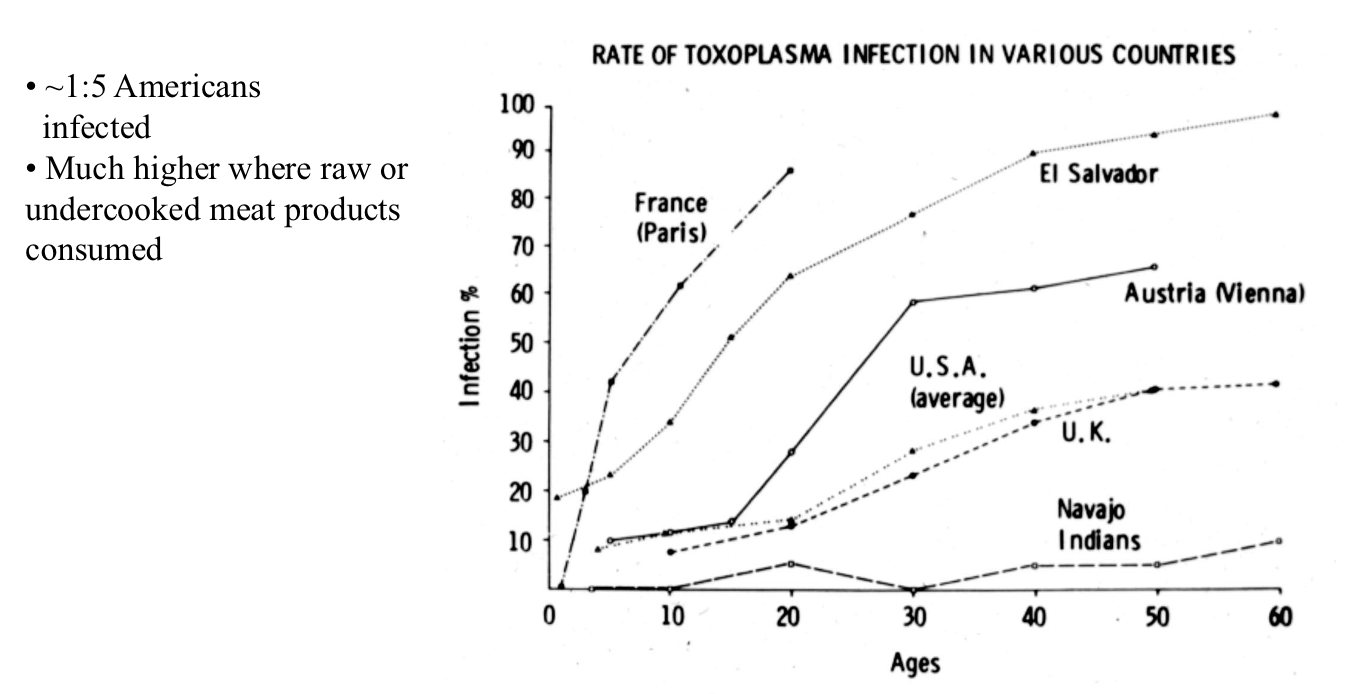
Acute Infection
oocysts from cat feces or tissue cysts in infected meat
infection initiates in intestine then disseminates throughout body
acute phase lasts 1-3 wks, usually asymptomatic or flu-like: fever lymphadenopathy headache myalgia
Latent Infection
Cytokines are critical for preventing reactivation of chronic infection
Periodic spontaneous cyst rupture stokes immune memory
Cysts often present in the brain
Toxoplasma Crossing the BBB
infect brain endothelial cells and grow inside cell
Eventually rupture out of cells and gain access to underlying brain tissue
Meta-analysis of 50 studies indicates a link between Toxoplasma gondii infection and
schizophrenia
bipolar disorder
Reactivation (Recrudescence)
bradyzoite (cyst) → tachyzoite (rapidly growing)
in immunodeficient or immunosuppressed individuals
Congenital Infection
Occurs only from primary infection during pregnancy
3-5% of babies from primary infected mothers have congenital effects
Transplacental transmission
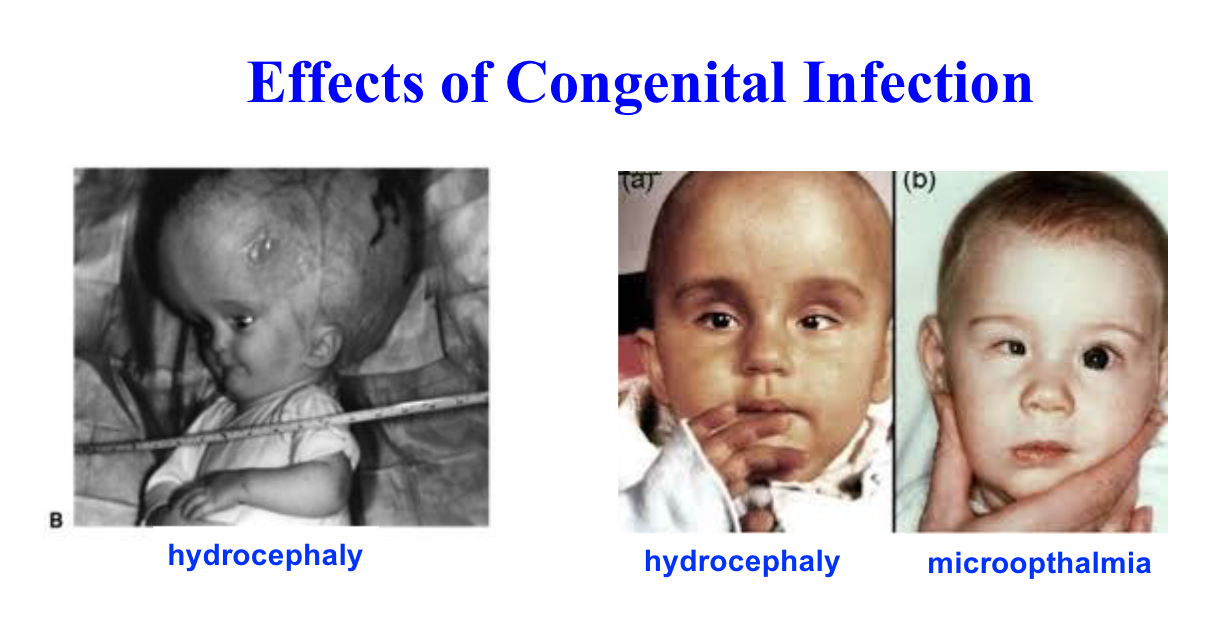
Sequelae of congenital infection
retinochoroiditis (iinflammation at back of eye)
microopthalmia
mental retardation
hydrocephaly
spontaneous abortion (miscarriage)
Ocular Toxoplasmosis
Reactivation or acute → Retinochoroiditis
Unilateral or bilateral vision impairment
Retinal lesions visible via opthalmoscopy
Local inflammation contributes to pathology
Recurrences common
Congenitally acquired infections can manifest at birth or reemerge later in life
oxoplasmosis treatments
many patients do not require treatment
pyrimethamine + sulfadiazine
OR
trimethoprim + sulfamethoxazole (bactrim)
OR
corticosteroids
pyrimethamine + sulfadiazine
both inhibit folic acid synthesis
often only required for immunocompromised patients
may require lifelong therapy at lowered dose if HIV+ and poorly controlled
trimethoprim + sulfamethoxazole (bactrim)
alternative medication (also inhibits folic acid production)
corticosteroids
for cerebral edema and ocular infections
Borrelia
Causative agent of Lyme Disease
in US Borrelia burgdorferi
B. burgdorferi grows in midgut of unfed tick.
Feeding induces migration to salivary glands in tick
Lyme Disease
Tick-borne disease transmitted from white-footed mice and white-tailed deer to man
Mouse host more relevant to human disease
symptoms of lyme disease
bells palsy (unilateral facial paralysis)
facial numbness
acute toothache radiating towards eyes and ears
Lyme Disease incubation period
3-30 days
Lyme Disease Stage I
Erythema migrans rash develops at site of tick bite
Starts at site of tick bite (2/3 of patients have rash). 5-50cm rash
Can culture organism from outer edge of rash
Early signs and symptoms: malaise, severe fatigue, headache, fever, chills, musculoskeletal pains, myalgias, lymphadenopathy (last for avg. of 4 wks)
Lyme Disease Pathogenesis
Spirochete enters skin and radiates out
Initial immune reaction suppressed by organism, allowing bacteria to multiply
B. burgdorferi enters blood and spreads throughout the body, flu-like symptoms
After a few weeks immune system holds infection in check
Early treatment with antibiotics can be effective
Lyme Disease Stage II
2-8 weeks after first signs of symptoms
Seen in 10-20% of pts; lasts days to months
Fatigue, headache, fever, malaise
Neurological symptoms: 10-20% of untreated pts severe headache, peripheral nerve neuropathy, facial nerve palsy, fatigue, difficulty concentrating
Cardiac dysfunction: 5%, atriventricular conduction block, myopericarditis, congestive heart failure
may require a pace-maker
Lyme Disease Stage III
Hallmark of Lyme Disease
begins ~6 months or years after skin rash, initial symptoms
60% develop this if untreated
Characterized by arthritis
Large joints generally involved
Chronic skin condition (acrodermatitis chronica atrophicans; more common in Europe)
Chronic disease
Lyme Disease - Prevention
Avoidance of ticks and their natural habitats
Protective clothing: long pants tucked into socks
Use of insect repellents
Check for ticks when return home
A recombinant vaccine was available
Withdrawn from market in 2002
accused of causing autoimmunity
FDA found no evidence of such
Withdrawn due to low demand for vaccine and potential lawsuits
Lyme Disease - Diagnosis
Diagnosis is clinical
Organism is hard to culture, but can use:
– Serology
– Polymerase chain reaction (PCR)
Lyme Disease - Treatment
Depends on stage
Erythema migrans - doxycycline drug of choice; alternative: amoxicillin or cefuroxime
Treatment lessens likelihood of late manifestations but arthritis, may still occur
Carditis, Neurologic, Arthritis - Ceftriaxone
B. burgdorferi-tick life-cycle
Tick eggs/larvae laid by females in spring are infected by feeding on
infected mice in summer/fall
Larvae develop into nymphs over winter
Nymph stage can transmit B. burgdorferi to dogs & humans
Most infections occur in late spring/early summer
90% of human cases from nymph stage
Adults ticks usually feed on deer, but can bite humans
How to remove a tick…
Grasp tick w/ fine tweezers/forceps as close to skin as possible
Pull straight and smoothly away from skin
Do NOT jerk or twist
Do NOT coat tick with anything or use heat to coax tick out
Infection of humans requires >48hrs of feeding
Using CRISPR to Make Mice Immune to Borrelia Infection
“Mice Against Ticks” project
Engineering white-footed mice to express antibodies protective against Lyme disease without requiring usual immune response
Antibody-encoding gene is introduced into mouse genome using CRISPR