4c + 4d Alkanes and alkenes
1/11
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
what is the general formula for alkanes
CnH2n+2
why are alkanes saturated hydrocarbons?
they contain no double C=C bonds so carbons are saturated because each carbon has its maximum of 4 single bonds
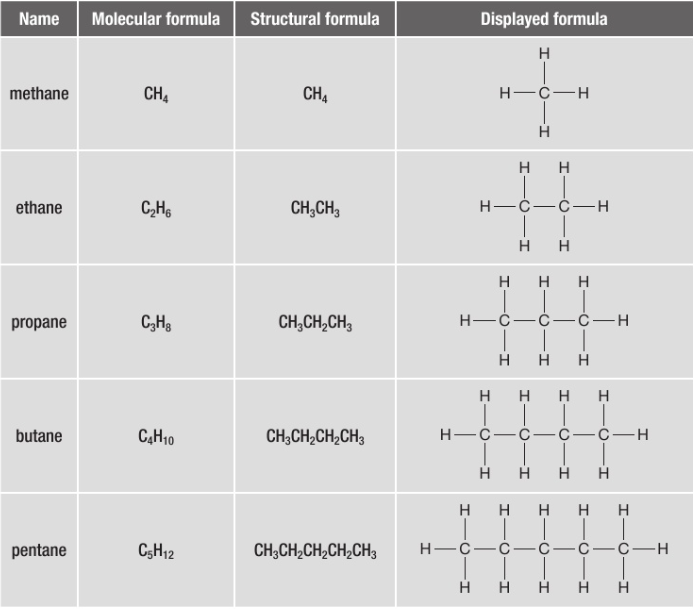
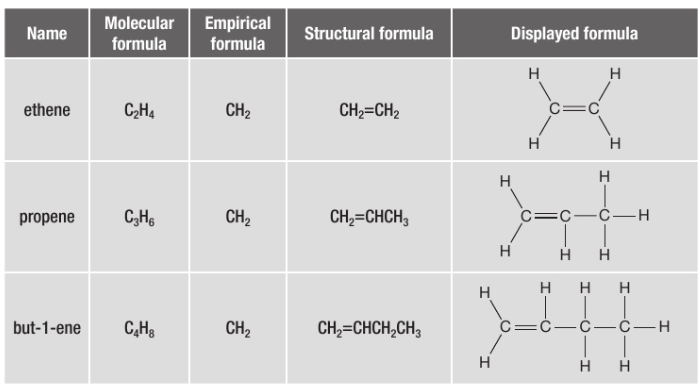
draw the first 5 alkanes
how do alkanes react with halogens?
they react under UV light such as sunlight
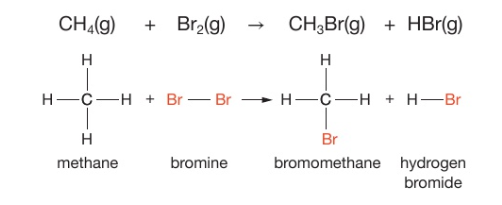
what do you see when an alkane and bromine gas react?
the mixture bromine and the alkane is orange but when reacted with an alkane under UV light, the solution is colourless
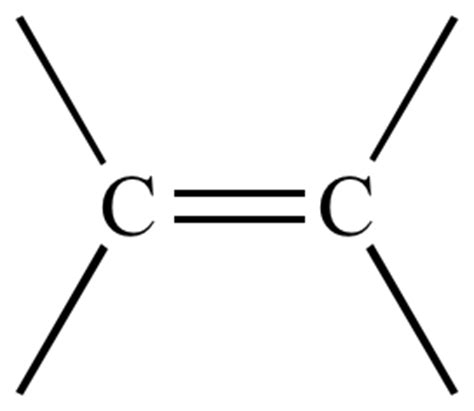
what is the functional group of alkenes?
what is the general formula for alkenes?
CnH2n
why are alkenes unsaturated hydrocarbons?
they contain one or more C=C double bonds
draw the first 3 alkenes
how do alkenes and bromine react?
addition reaction
alkene + bromine → dibromoalkane
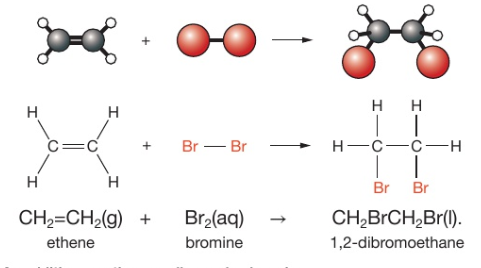
what happens when alkenes and bromine react?
the removal of the C=C double bond
how can alkanes and alkenes be distinguished?
alkenes react with bromine water → turns from orange to colourless
alkanes DO NOT react with bromine water → it remains orange