Chirality
1/19
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
20 Terms
What is meant by the term stereoisomerism
Molecules with the same structural formula but different spacial arrangement of atoms
What are the types of stereoisomerism
Geometric or optical
How do optimal isomers occur
When two compounds have the same molecular formula but are not superimposible on each other
What are enantiomers
When a compound contains a carbon atom bonded to four different groups or atoms, existing in two forms which are non-superimposable mirror images of each other
What is a carbon atom with four different groups bonded to it called
A chiral centre
How do two chiral isomers affect plane polarised light
By rotating the plane in opposite directions
What is an optically inactive mixture containing enantiomers and how does it affect plane polarised light
An equimolar or racemic mixture/racemate when enantiomers are in 1:1
Does not rotate light as equal and opposite rotations cancel
How is plane polarised light made
By passing light through a polarising filter (a polaroid) and oscillates in only one plane
What are polarimeters used for
To determine whether a sample interacts with plane polarised light
What are the 6 parts of the polarising process to determine the enantiomers
Lamp → monochromator filter → polarising filter → sample cell → analysing filter → detector
What is an Sn1 reaction
When there is only one species in the rate determining step
What is a Sn2 reaction
Where there are two species involved in the rate determining step
Which out of 1˚2˚3˚ has the fastest rate of reaction in a Sn2 nucleophilic substitution reactiob
1˚>2˚>>3˚
Explain how the concept of steric hinderance explains the rate 1˚>2˚>>3˚in a Sn2 reaction
A tertiary haloalkane is surrounded by 3 alkyl groups which inhibit the attack from a nucleophile (steric hinderance) so nucleophile is unable to attack the carbon in C-X bond as readily as it could for primary
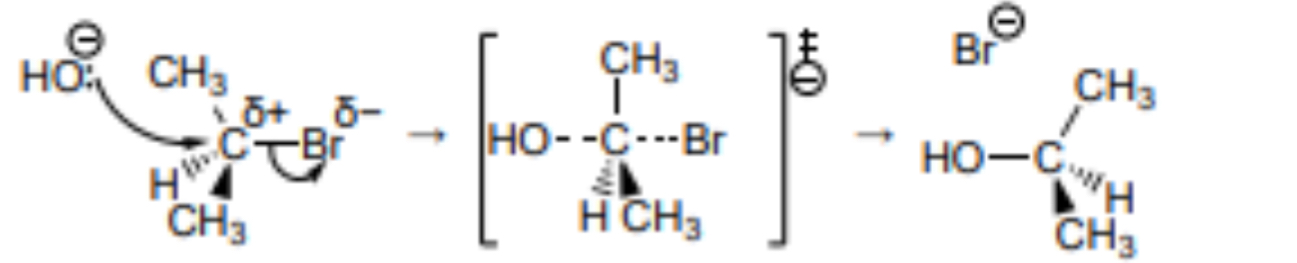
Outline the Sn2 mechanism for the reaction between 2-bromopropane and NaOH
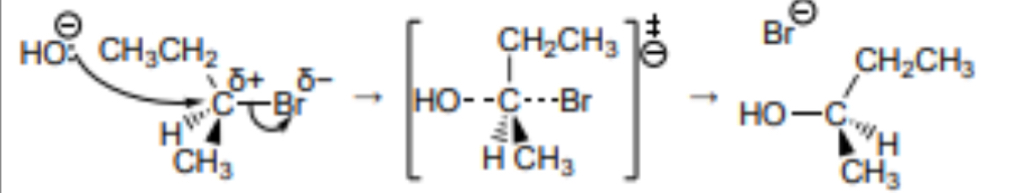
Outline the Sn2 mechanism for the reaction between 2-bromobutane and NaOH
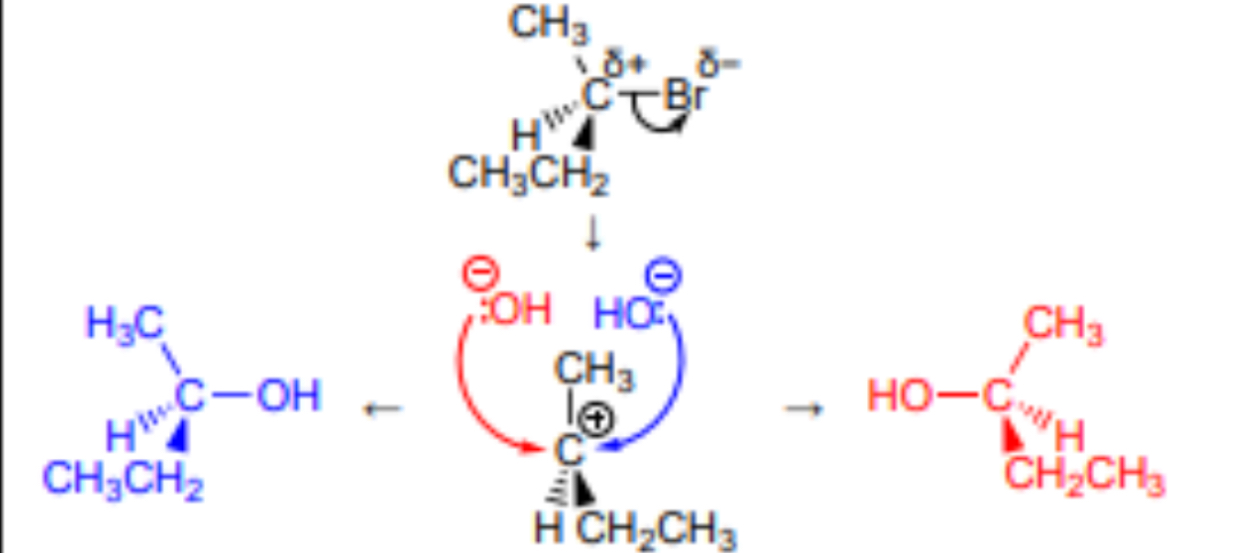
Outline the Sn1 mechanism for the reaction between 2-bromopropane and NaOH
Which out of 1˚2˚3˚ has the fastest rate of reaction in a Sn2 nucleophilic substitution reactiob
3˚>>2˚>1˚
Explain how the stability of carbocations explains the rates 3˚>>2˚>1˚ In a Sn1 reaction
Reaction proceeds through a carbocation intermediate. Tertiary carbocations are most stable than primary or secondary due to three alkyl groups resulting in positive inductive effects
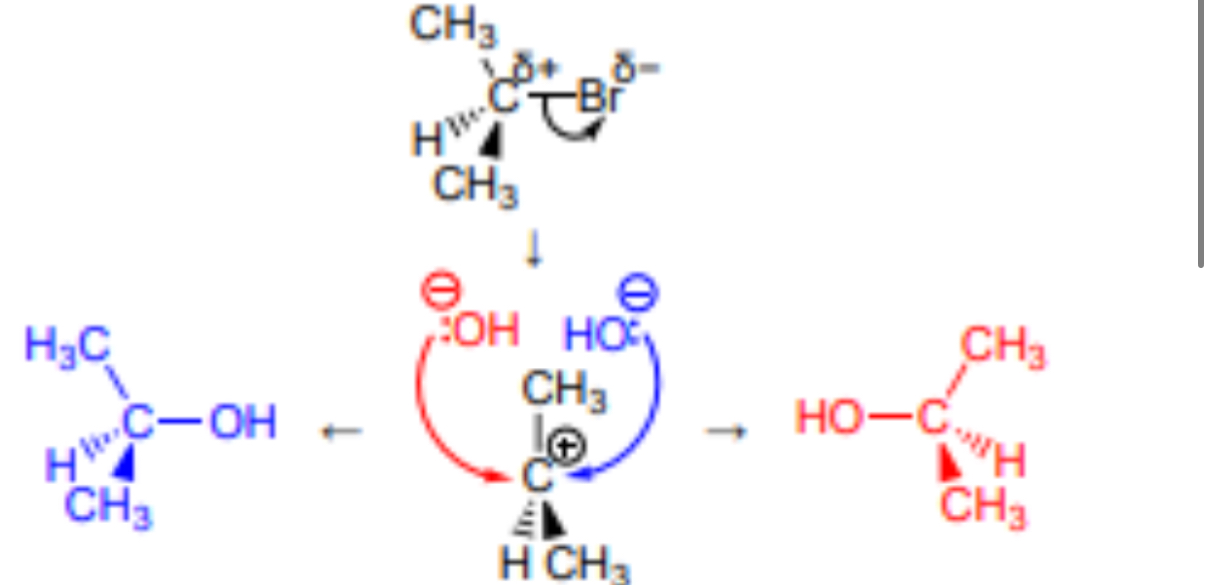
Outline the Sn1 mechanism for the reaction between 2-bromobutane and NaOH