Aldehydes, Ketones and Carboxylic Acids
1/56
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
57 Terms
Carbonyl Compounds
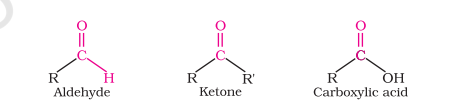
Aldehydes (R-CHO)
Ketones (R-CO-R’)
Carboxylic Acids (R-COOH)
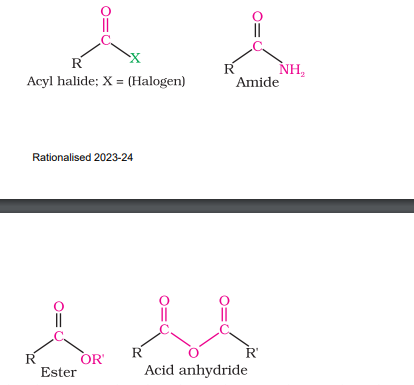
Carboxylic Acid Derivatives
Esters (R-COOR)
Acyl Chloride (R-COCl)
Amide (R-CONH₂)
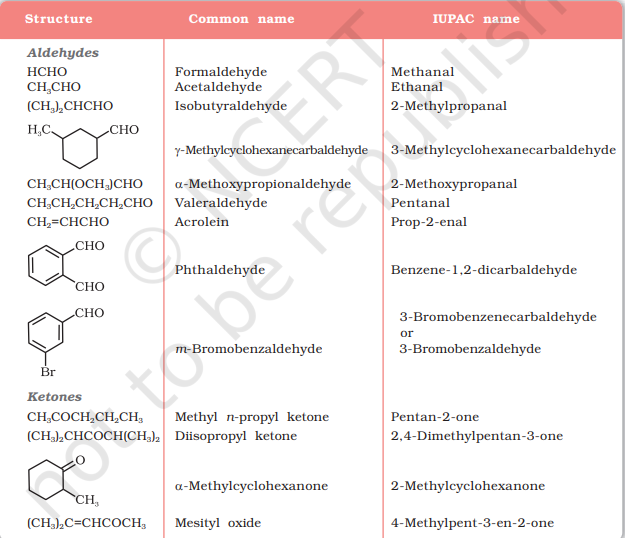
Common and IUPAC Names of Some Aldehydes and Ketones
Preparation of Aldehyde
Rosenmund reduction
Stephen reaction
From hydrocarbons:
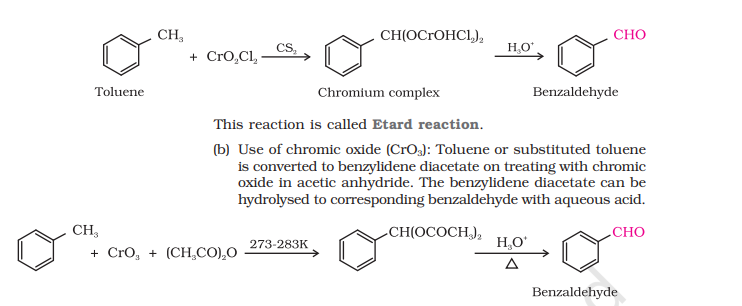
Etard Reaction
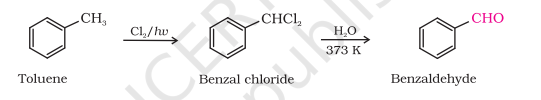
By side chain chlorination followed by hydrolysis
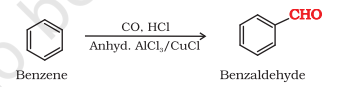
Gatterman Koch Reaction
Ozonolysis
Hydration of Alkynes
Catalytic Dehydration
Rosenmund Reduction (from acyl chloride)
R-COCL → [H₂, Pd-BaSO₄] R-CHO
C₇H₅ClO (benzoyl chlrodie) → [H₂, Pd-BaSO₄] C₆H₅CHO (benzaldehyde)
CH₃COCl → [H₂, Pd-BaSO₄] CH₃CHO
The role BaSO₄ is to prevent further reduction of aldehydes to 1ᵒ alcohol (since COOH and COOH derivatives (acyl chlorides) on reduction, convert to 1ᵒ alcohol)
![<p>R-COCL → [H<span>₂, Pd-BaSO₄] R-CHO</span></p><p><span>C₇H₅ClO (benzoyl chlrodie) </span>→ [H₂, Pd-BaSO₄] C<span>₆</span>H₅CHO (benzaldehyde)<br><br>CH<span>₃COCl → </span>[H₂, Pd-BaSO₄] CH₃CHO</p><p>The role BaSO₄ is to prevent further reduction of aldehydes to 1<span>ᵒ alcohol (since COOH and COOH derivatives (acyl chlorides) on reduction, convert to 1</span>ᵒ alcohol)</p><p></p>](https://knowt-user-attachments.s3.amazonaws.com/79c7baa3-0a8f-4b90-88de-b4f637db4c73.png)
Stephen Reaction
RCN + SnCl₂ + HCl → RCH=NH →[H₂O]RCHO
CH₃-CH₂-CN→ [SnCl₂+HCl] CH₃-CH₂-CH=NH→ [H₃O⁺] CH₃-CH₂-CHO
CH₂=CH-CN → [DIBAL-H] CH₂=CH-CH=NH → CH₂=CH-CHO (not stephen’s reaction)
CH₂=CH-CN → [SnCl₂+HCl] CH₃-CH₂-CHO
![<p>RCN + SnCl₂ + HCl → RCH=NH →[H₂O]RCHO</p><p>CH<span>₃-</span>CH₂-CN→ [SnCl₂+HCl] CH₃-CH₂-CH=NH→ [H₃O<span>⁺] </span>CH₃-CH₂-CHO</p><p></p><p></p><p>CH₂=CH-CN → [DIBAL-H] CH₂=CH-CH=NH → CH₂=CH-CHO (not stephen’s reaction)</p><p>CH₂=CH-CN → [SnCl₂+HCl] CH₃-CH₂-CHO</p><p></p>](https://knowt-user-attachments.s3.amazonaws.com/543d4c3c-2ad5-4139-9782-b0dcfba8c667.png)
3) Etard Reaction
4) By Side Chain Chlorination Followed By Hydrolysis
5) Gatterman Koch Reaction
6) Ozonolysis
see cw
7) Hydration of Alkynes
R-C≡C-R→[H₂O,H⁺] R-C(OH)=CH-R→ [Proton shift'/Tautomerism] R-C(O)-CH₂-R
CH≡CH→[H₂O,H⁺] CH(OH)=CH₂ → [H⁺ Shift] CH₃CHO
8)Catalytic Dehydration
R-CH₂-OH → [Cu, 573K] R-CHO
R-CH(OH)-R’ → [Cu, 573K] R-C(O)-R’
Preparation of Ketones
From Grignard Reagents
From benzene (Friedel Craft’s Acylation)
From Acyl Chlorides
From Grignard Reagents
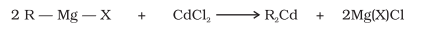
From Benzene (Friedel Craft’s Acylation)
anhy.AlCl₃
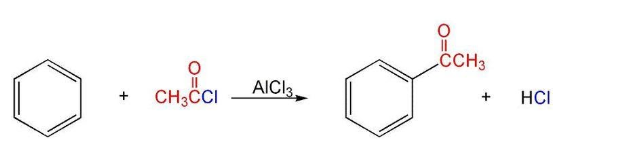
From Acyl Chlorides

Physical Properties
Methanal is a gas and ethanal is a volatile liquid while other aldehydes are mostly liquids and other soft solids
Comparatively, aldehydes have higher boiling points due to dipole-dipole interactions but have lesser boiling points than alcohols due to the absence of inter-molecular hydrogen bonding
The lower members of aldehydes and ketones are completely miscible in water as they can form hydrogen bond
with water and solubility decreases with an increase in size
The lower members have a sharp pungent odor but as the size increases, they become more and more fragrant. Hence, they are naturally used as flavouring agents and perfume blends.
Chemical Properties
Nucleophilic Addition Reaction:
with HCN
with NAHSO₃
with Alcohol
Alcohol + Ketones
with Diols
with Grignard Reagents
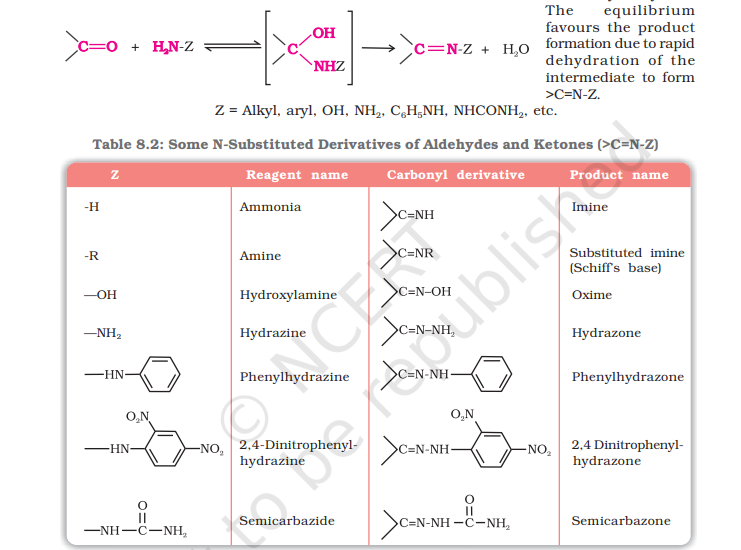
with Ammonia and its derivatives
Nucleophilic Addition Reaction
always remove hydrogen and consider the rest as nucleophile
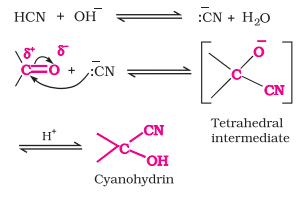
with HCN
Q; Cyanohydrin of Acetaldehyde
(R)C(R’)=O + HCN → (CN)(R)C(R’)(OH)
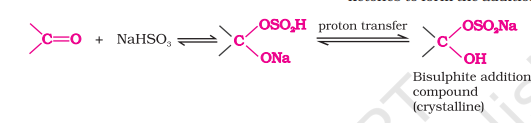
with NAHSO₃
can directly give the final product, no need to write the middle product
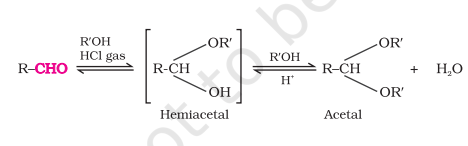
with Alcohol
Q: Acetal and semiacetal of acetaldehyde and ethanol
same can be done for ketone
Alcohol + Ketones
see note
with Diols
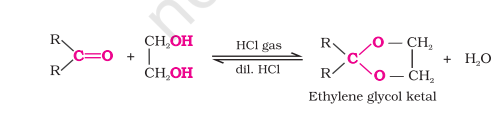
with Grignard Reagents
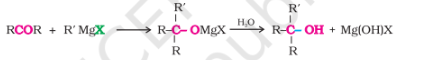
with Ammonia and its derivatives
Aldol Condensation
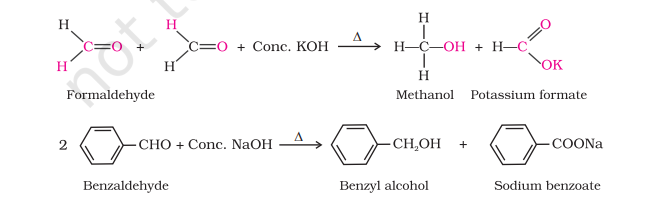
Cannizzaro reaction
Aldehydes which do not have an α-hydrogen atom, undergo self oxidation and reduction (disproportionation) reaction on heating with concentrated alkali. In this reaction, one molecule of the aldehyde is reduced to alcohol while another is oxidised to carboxylic acid salt.
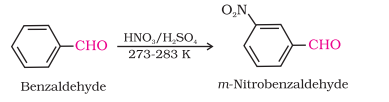
Electrophilic Substitution Reaction
carbonyl group acts as a meta-directing group.
Order of Reactivity of Aldehydes and Ketones towards Nucleophilic Addition
Aldehydes are more reactive than ketones due to lesser +I effect and lesser steric hindrance of aldehydes than ketones.
Lighter aldehydes are more reactive than heavier aldehydes due to lesser +I effect and lesser steric hindrance.
Lighter ketones are more reactive than heavier ketones.
Benzaldehydes are less reactive than aldehydes due to resonance in the aromatic ring, the electrophilicity of carbonyl carbon is decreased.
Benzaldehydes with electron-withdrawing groups (e.w.g) are more reactive than benzaldehydes with electron-donating groups (e.d.g).
Tests for Aldehydes and Ketones
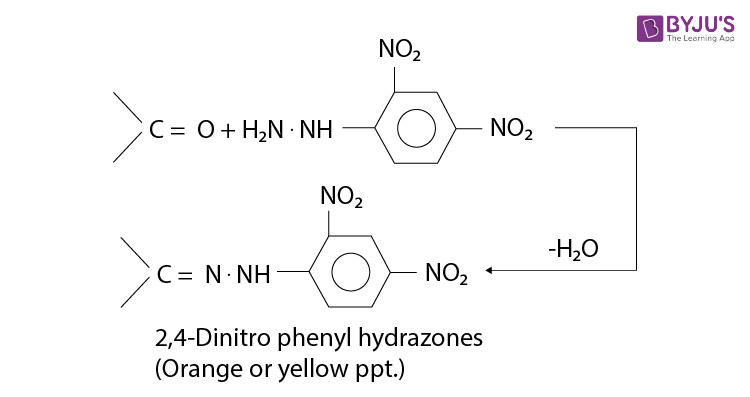
2,4-Dinitrophenyl Test
Tollen’s Test
Fehling’s Test
Iodoform Test
1) 2,4-Dinitrophenyl Test
Test for carbonyl carbon
Orange precipitate is formed
2) Tollen’s Test
Test for aliphatic/aromatic aldehydes
Tollen’s Reagent: Ammoniacal silver nitrate solution Ag(NH3)2OH
Oxidation takes place
Bright silver mirror is formed
R-CHO+Ag⁺ → R-COO⁻ + Ag↓
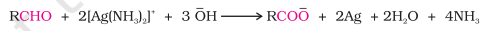
3) Fehling’s Test
Test for Aliphatic Aldehyde
Does not work for aromatic
Fehling’s Reagent A: aq.CuSO₄
Fehling’s Reagent B: Na/K tartarate
Oxidation takes place

4) Iodoform Test
Test for methyl carbonyl (compounds with CH₃-C(O)- group/acetyl group)
Reagent: NaOI or NaOH + I₂
R-C(O)-CH₃ + NaOI → R-COONa + CHI₃↓
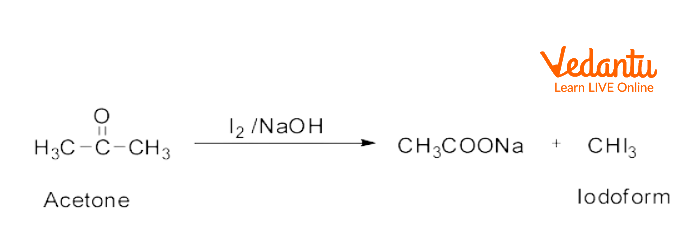
Uses of Aldehydes and Ketones
Formaldehyde is well known as formalin (40%) solution used to preserve biological specimens and to prepare bakelite, urea-formaldehyde glues and other polymeric products.
Benzaldehyde is used in perfumery and in dye industries.
Carboxylic Acids
-C(O)-O - carboxyl
Some higher members of aliphatic carboxylic acids (C₁₂ – C₁₈) known as fatty acids, occur in natural fats as esters of glycerol.
Names and Structures of Some Carboxylic Acids
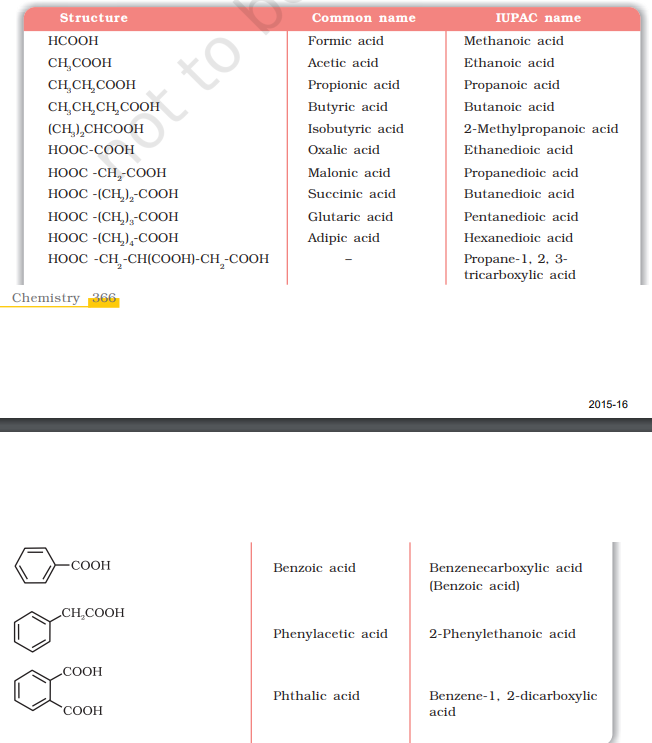
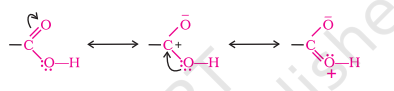
Structure of Carboxyl Group
The carboxylic carbon is less electrophilic than carbonyl carbon because of the possible resonance structure shown in image
Preparation of Carboxylic Acids
From alcohols (by oxidation)
From Alkyl Benzene
From Grignard Reagent
From Acid Anhydrides
From Esters
Preparation of Carboxylic Acids
1) From Alcohols (by oxidation)
Primary alcohols are readily oxidised to carboxylic acids with common oxidising agents such as potassium permanganate (KMnO₄) in neutral, acidic or alkaline media or by potassium dichromate (K₂Cr₂O₇ ) and chromium trioxide (CrO₃) in acidic media (Jones reagent).
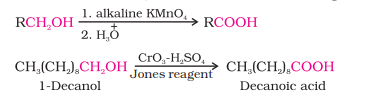
Preparation of Carboxylic Acids
2) From Alkyl Benzene
The entire side chain is oxidised to the carboxyl group irrespective of length of the side chain
Primary and secondary alkyl groups are oxidised in this manner while tertiary group is not affected.
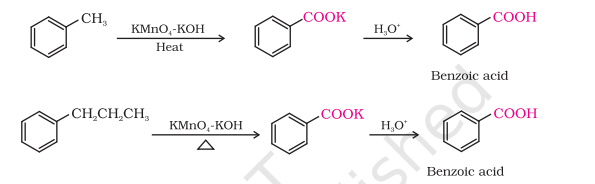
Preparation of Carboxylic Acids
3) From Grignard Reagent
Carbon dioxide is in the form of solid dry ice
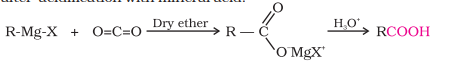
Preparation of Carboxylic Acids
4) From Acid Anhydrides
CH₃COOCOCH₃ → [H₃O⁺] 2CH₃COOH
(CH₃CO)₂O
Preparation of Carboxylic Acids
5) From Esters
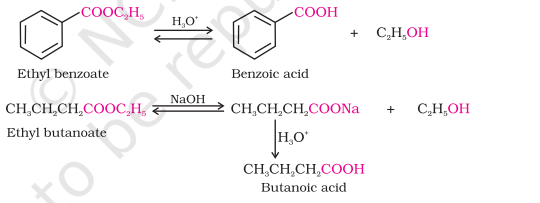
Physical Properties
Aliphatic carboxylic acids upto nine carbon atoms are colourless liquids at room temperature with unpleasant odours
Carboxylic acids are higher boiling liquids than aldehydes, ketones and even alcohols of comparable molecular masses due to more extensive association of carboxylic acid molecules through intermolecular hydrogen bonding
Simple aliphatic carboxylic acids having upto four carbon atoms are miscible in water due to the formation of hydrogen bonds with water. The solubility decreases with increasing number of carbon atoms.
Benzoic acid, the simplest aromatic carboxylic acid is nearly insoluble in cold water.
Chemical Properties
1) Acidic Character
2) Esterification
3) Dehydration
4) Chlorination
5) Addition of Ammonia
6) Hell-Volhard-Zelinsky reaction (Halogenation)
7) Electrophilic Subsitution
8)Reduction
9) Decarboxylation
Chemical Properties
1) Acidic Character (Reactions with metals and alkalies)
2R-COOH + 2Na → 2R-COONa⁺ + H₂
R-COOH + NaOH → R-COONa⁺ + H₂O
Bicarbonate Test (Test for carboxylic acids):
R-COOH + NaHCO₃ → R-COONa⁺ + H₂O + CO₂↑ (brisk effervescence)
Carboxylic acids dissociate in water to give resonance-stabilised carboxylate anions and hydronium ions.
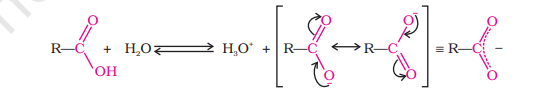
Order of Acidic Character
HCOOH > CH₃COOH
CH₃COOH < CH₂(Cl)COOH < (Cl)CH₂(Cl)COOH
Acidic character ∝ [H⁺] ∝ 1/pH ∝ Ka ∝ i/pKa
The electron-withdrawing effect of the following groups in increasing acidity order is
Ph < I < Br < Cl < F < CN < NO2 < CH₂X < CHX₂ < CX₃
X - any e.w.g
Chemical Properties
2) Esterification
R-COOH = R’-OH → RCOOR’ + H₂O
CH₃COOH + CH₃OH → CH₃COOCH₃ + H₂O
Chemical Properties
3) Dehydration
2R-COOH → [P₂O₅] R-C(O)-O-C(O)-R + H₂O
2CH₃COOH → [P₂O₅] CH₃-C(O)-O-C(O)-CH₃ + H₂O
R-COOH+ R’COOH → [P₂O₅] R-C(O)-O-C(O)-R’ + H₂O
CH₃-COOH + C₆H₅COOH → [P₂O₅] C₆H₅-C(O)-O-C(O)-CH₃ + H₂O
![<p>2R-COOH → [P<span>₂O₅] R-C(O)-O-C(O)-R + </span>H₂O</p><p><span>2CH</span>₃COOH → [P₂O₅] CH₃-C(O)-O-C(O)-CH₃ + H₂O</p><p>R-COOH+ R’COOH → [P₂O₅] R-C(O)-O-C(O)-R’ + H₂O</p><p>CH₃-COOH + C<span>₆H₅COOH → [</span>P₂O₅] C₆H₅-C(O)-O-C(O)-CH₃ + H₂O </p>](https://knowt-user-attachments.s3.amazonaws.com/89c5aeba-997a-4498-96fd-8973f52ebd80.png)
Chemical Properties
4) Chlorination
RCOOH + PCl₅ → RCOCl + POCl₃ + HCl
3RCOOH + PCl₃ → 3RCOCl + H₃PO₃
RCOOH + SOCl₂ → RCOCl + SO₂ + HCl
Chemical Properties
5) Addition of Ammonia
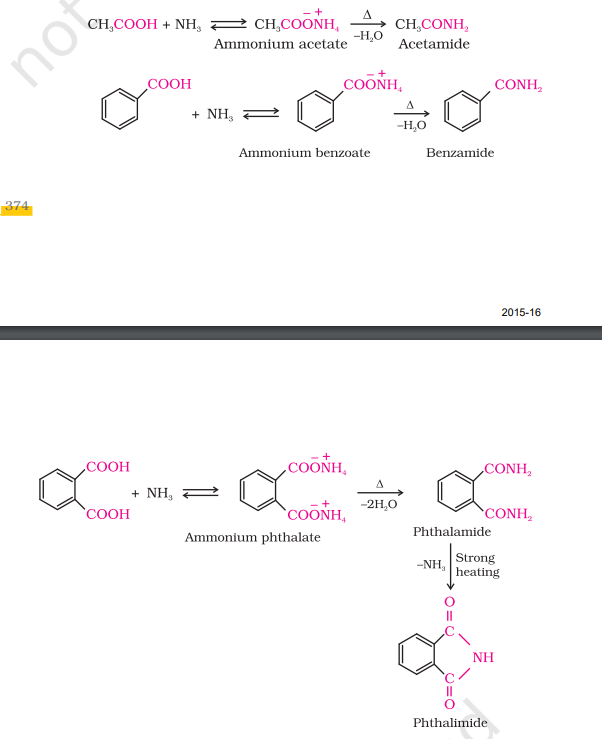
Chemical Properties
6) Hell-Volhard-Zelinsky reaction (Halogenation)
CH₂(Cl)-COOH +HCl
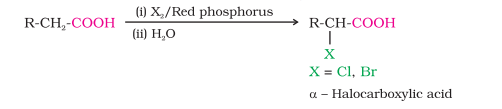
Chemical Properties
7) Electrophilic Substitution
They do not undergo Friedel-Crafts reaction (because the carboxyl group is deactivating and the catalyst aluminium chloride (Lewis acid) gets bonded to the carboxyl group).
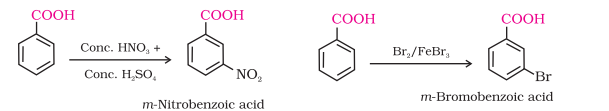
Chemical Properties
8) Reduction

Chemical Properties
9) Decarboxylation
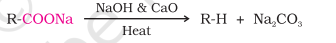
NaOH + CaO = Soda Lime