Building blocks VII Amino Acids
1/14
Earn XP
Description and Tags
Recall the biochemical principles underlying the structure of the amino acids, including modifications • Be aware of the principles of the classification of amino acids • Describe how proteins consist of amino acids, and explain the terms primary, secondary, tertiary and quaternary structure
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
amino acids
DNA → RNA → protein
first discovered in 19th century
> 700 exist in nature but only 22 building blocks for proteins in cells
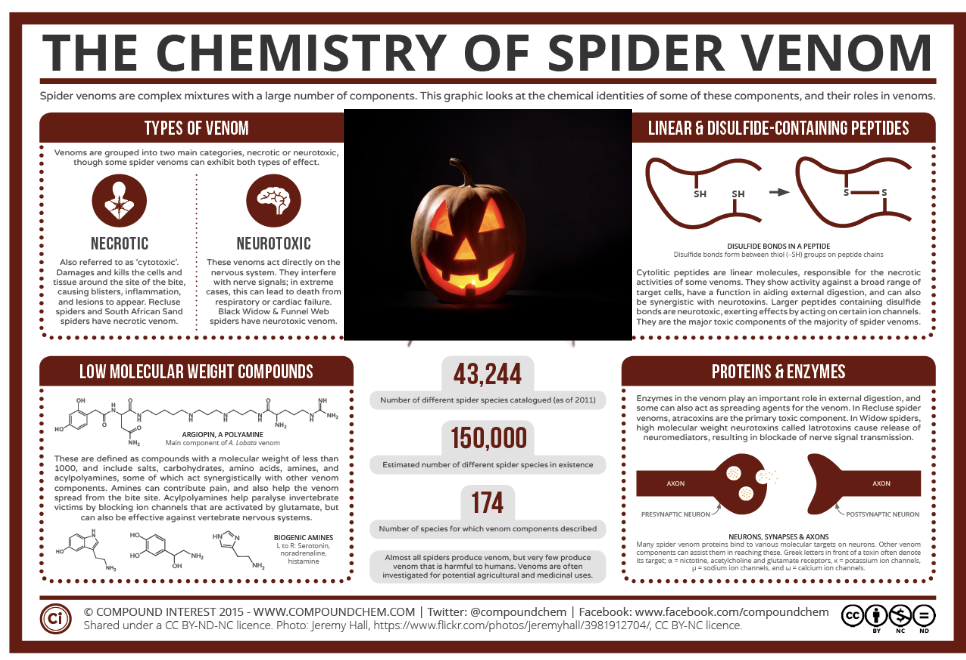
Structure:
Amino group - one side of polypeptide chain in proteins has this group exposed. it is called the N-terminus end
Carboxyl group - other side of the polypeptide chain in proteins has this group exposed. it is called the C-terminus end
Side chain -defines chemical characteristics of the amino acid
what are the classifications of AA
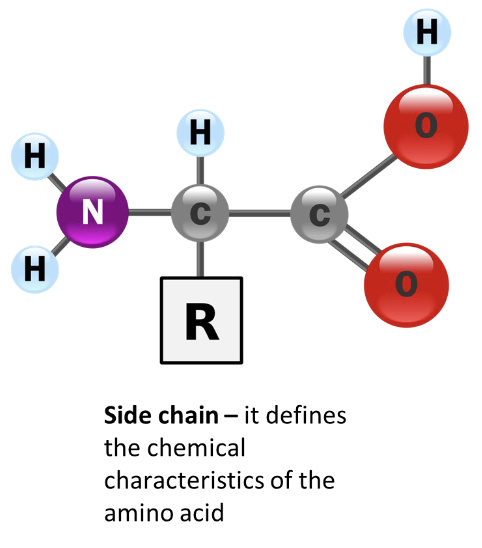
1. The chemical characteristics of their side chains
• Polar
• Non-polar
• Charged
• Non-charged
• Aliphatic
• Aromatic
2. Synthesis
• Proteinogenic
• Non-proteinogenic
3. Essential/Non-essential
• Essential
• Conditionally essential
• Non-essentia
what are the chemical characteristics of AA side chains?
polar
non-polar
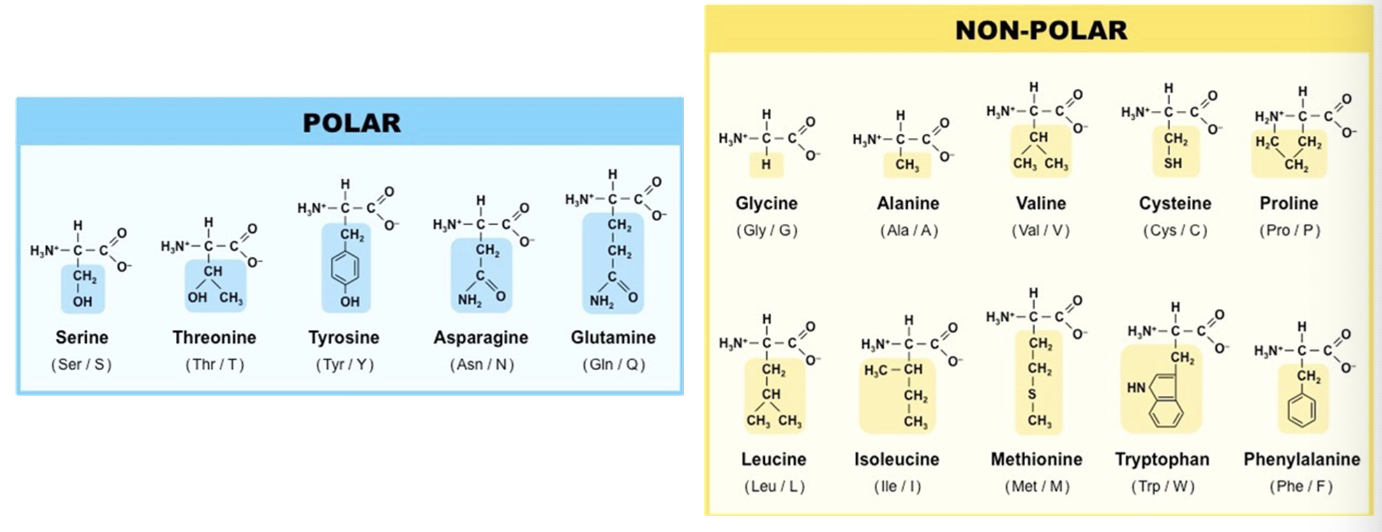
what does it mean if they have positive or negative charge?
+ charge = basic
- charge = acidic
Y, W and F can also be classified as aromatic because of the aromatic ring they carry
what are proteinogenic AA
they are the building blocks of proteins
20 are encoded by triplets of the genetic code
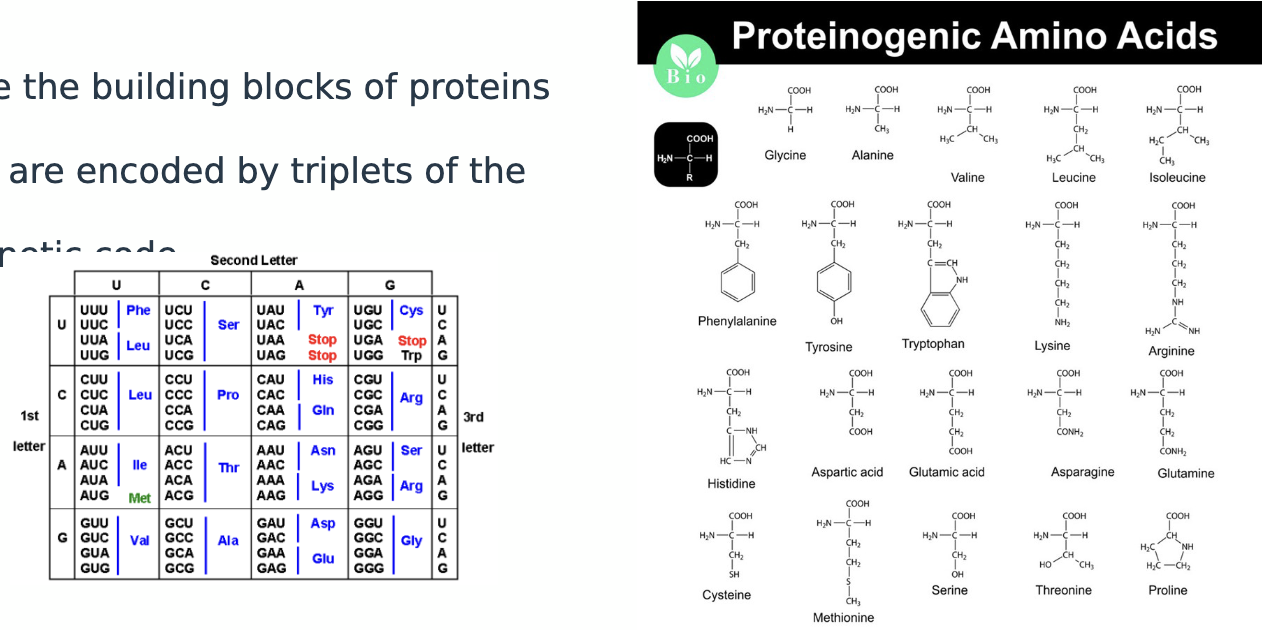
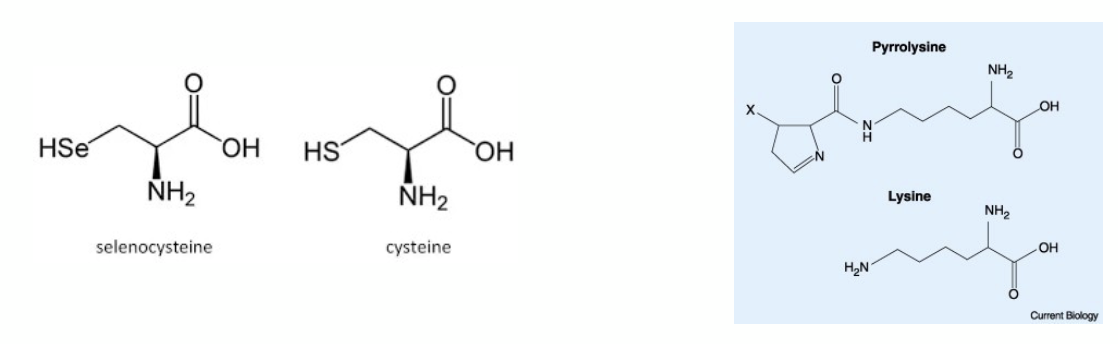
which 2 codons are incorporated into proteins through specific mechanisms and how?
selenocysteine
pyrrolysine
they both contain a structural change in the mRNA sequence allowing a STOP codon not to be recognised as such by a specific tRNA
what are non proteinogenic amino acids?
Have other biological functions beyond forming proteins
• GABA (neurotransmitter)
• Carnitine (transport of fatty acids in the blood)
• Citrulline and ornithine (role in urea cycle)
Are not produced directly and in isolation by standard cellular machinery
• Modified by post-translational modification of the protein in which
they are embedded
• occurring post-translationally, once the protein has already formed
• often linked to the function/activity of the protein
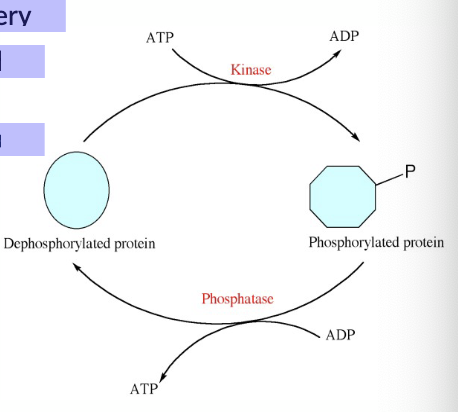
Common modifications:
• Phosphorylation (by kinases)
• Dephosphorylation (by phosphatases)
• Methylation
• Acetylation
• Hydroxylation
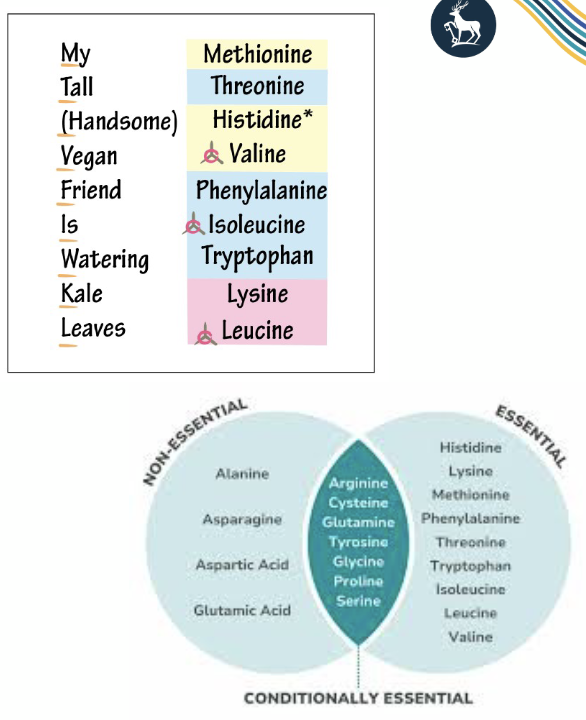
what are essential/non-essential AA?
Essential
• His, Ile, Leu, Lys, Met, Phe,
Thr, Trp and Val
• can’t be synthetized by the body and must be obtained through the diet
Conditionally essential
• Arg, Cys, Gln, Tyr, Gly, Pro, and Ser
• In condition of stress a surplus of these amino acids need to be uptaken through the diet to cope with body demand
Non essential
• Ala, Arg, Agn, Asp, Cys, Glu, Gln, Gly, Pro, Ser, and Tyr
• Can be synthetized by the body
what is AA deficiency in context?
Herbivores
hay/straw/haylage: high levels of LIGNIN, which reduces the absorption of nutrients
Decreased levels of essential AA such as Lys
Animal with normal weight but with collateral issue — Hooves, Coat, appear damaged/compromised
Can reduce the ability of immune system to fight infection
Cats
taurine is another amino acid
essential for cats
must be obtained through dietary enrichment (cats cant produce it themselves)
lack of it leads to serious health problems like retinal degeneration, heart conditions and reproductive problems
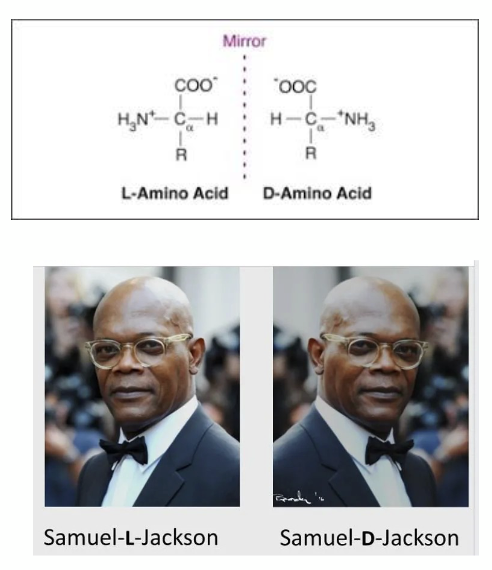
what are enantiomers?
• All amino acids exist in two optically asymmetric forms (mirror images) except glycine
• These are called enantiomers
• L- and D- enantiomers exist
• AA in proteins are almost exclusively L form
• Reflecting this the L is mostly omitted
• In nature some D – form AA found in cell walls of bacteria and in some antibiotics but these are not synthesised in the ribosome
• Term enantiomer can also apply to pharmacology
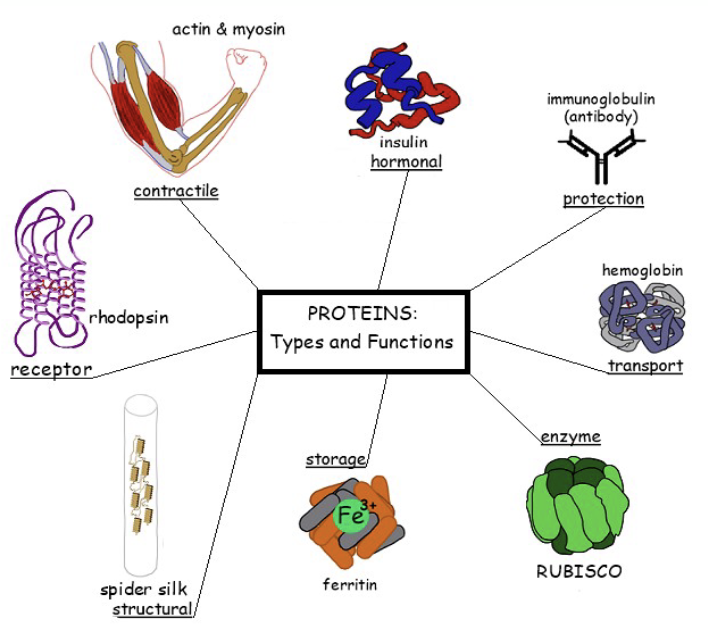
what are the structure and function of proteins?
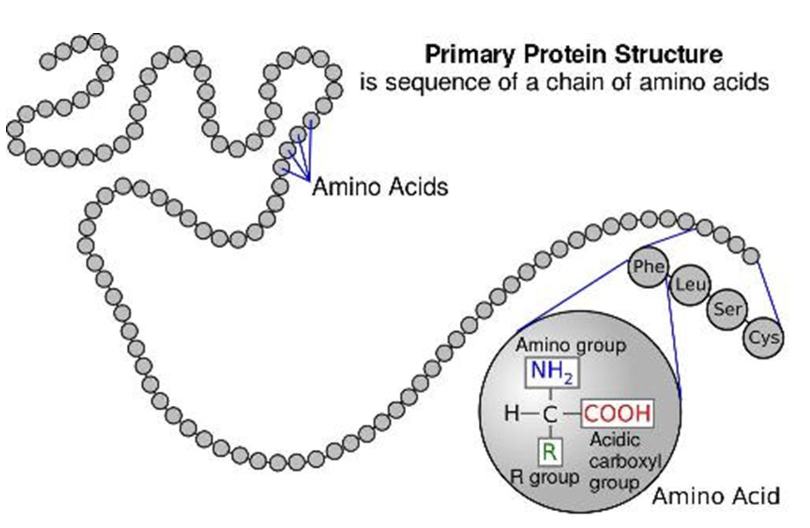
what is the primary structure of primary protein structure?
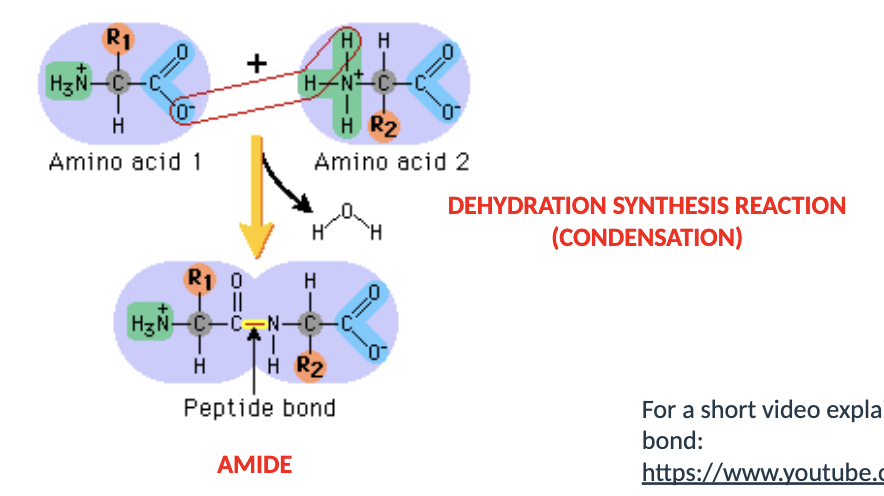
what is the polypeptide chain?
amino acids are bound together through peptide (amide) bonds
a protein is a polypeptide chain
they are joined through dehydration synthesis reaction (also known as condensation)
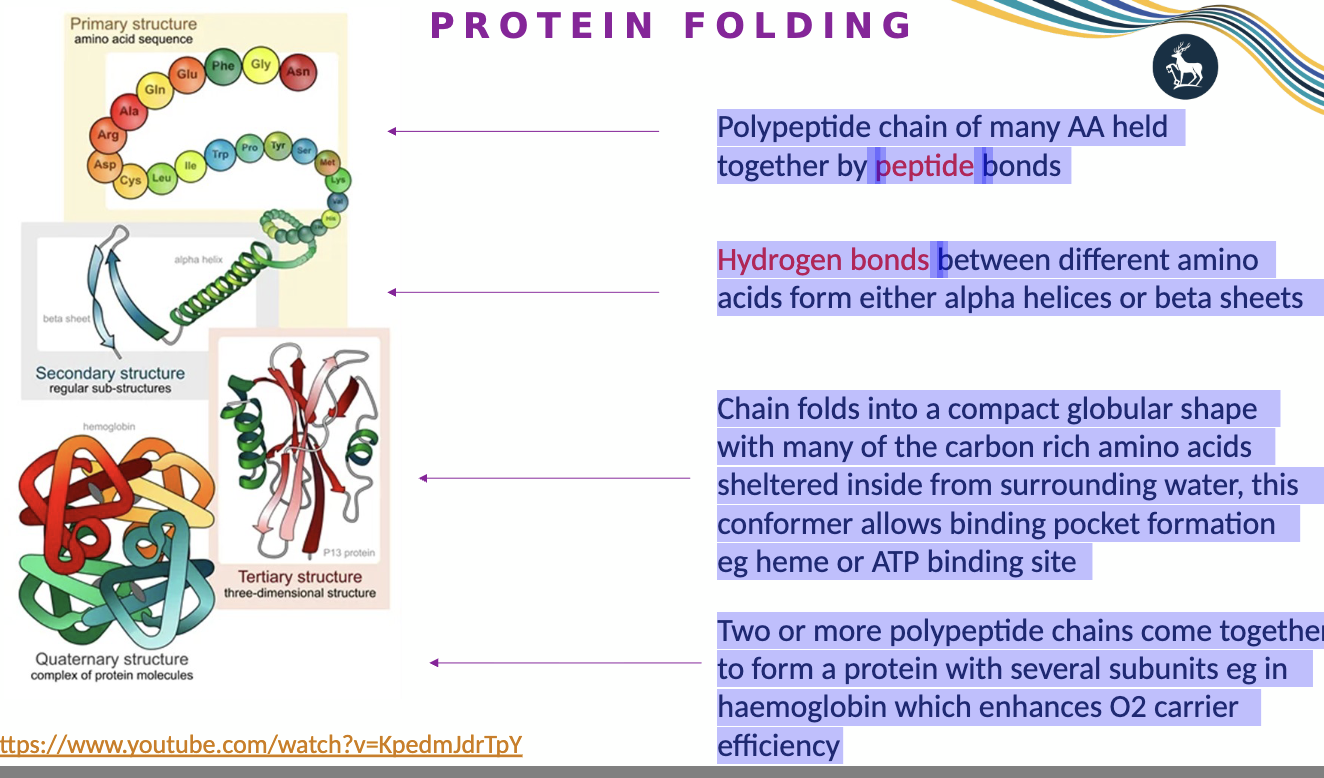
what is protein folding?
Polypeptide chain of many AA held together by peptide bonds
Hydrogen bonds between different amino acids form either alpha helices or beta sheets
Chain folds into a compact globular shape with many of the carbon rich amino acids sheltered inside from surrounding water, this conformer allows binding pocket formation e.g. heme or ATP binding site
Two or more polypeptide chains come together to form a protein with several subunits eg in haemoglobin which enhances O2 carrier efficiency
what are bioactive peptides?