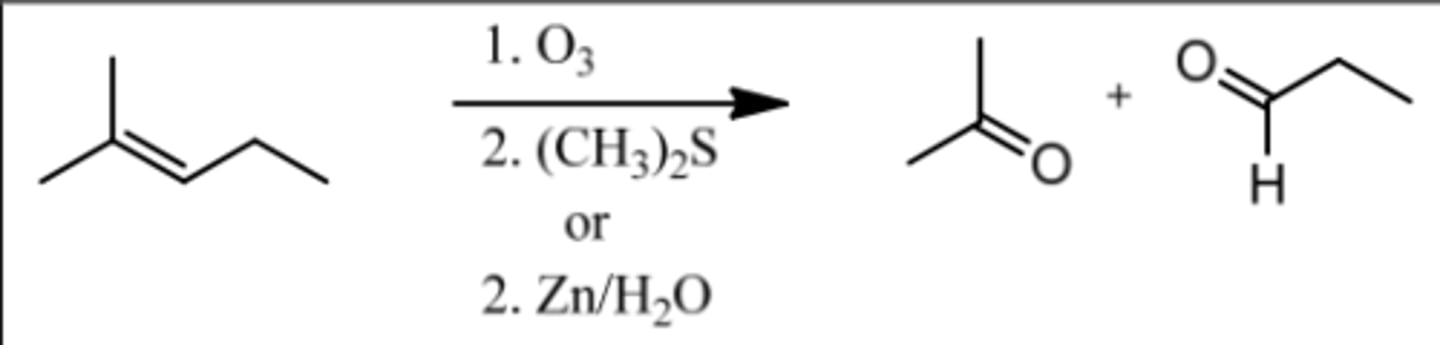Organic Chemistry (Alkene Reactions)
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
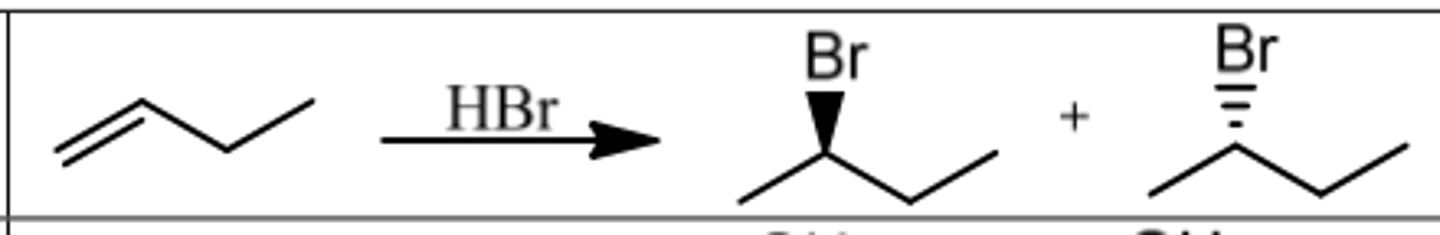
Reaction: Hydrohalogenation
Reagents: HBr (or HCl, HI)
What's Added: H⁺ & Br⁻
Regioselectivity: Markovnikov
Sterioselectivity: -
Intermediate: Carbocation
Rearrangements: Possible
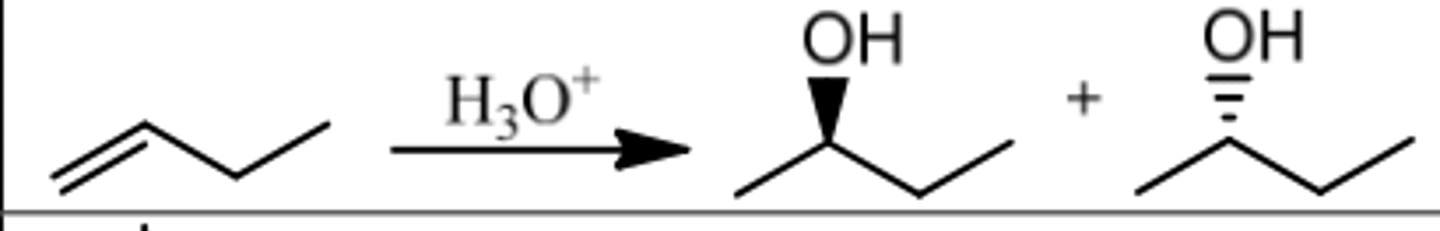
Reaction: Hydration
Reagents: H₃O⁺
What's Added: H⁺ & OH⁻
Regioselectivity: Markovnikov
Sterioselectivity: -
Intermediate: Carbocation
Rearrangements: Possible
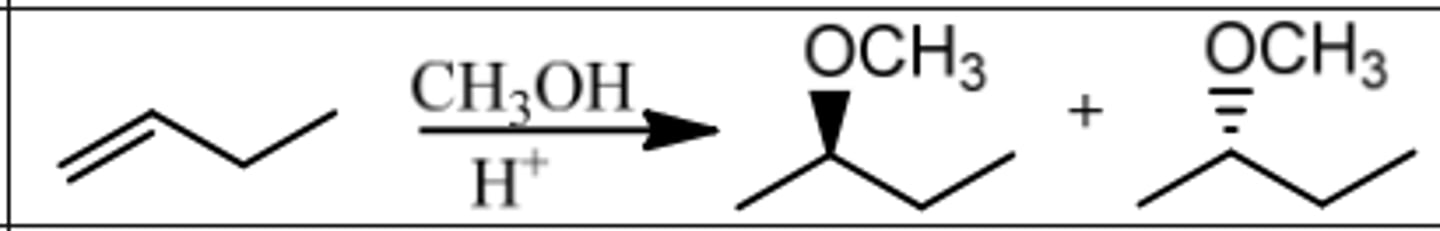
Reaction: Addition of Alcohol
Reagents: H⁺, ROH
What's Added: H⁺ & OR⁻
Regioselectivity: Markovnikov
Sterioselectivity: -
Intermediate: Carbocation
Rearrangements: Possible
Reaction: Bromination
Reagents: Br₂/CCl₄ (or Cl₂)
- CCl₄ & Cl₂ are Inert
What's Added: Br⁺ & Br⁻
Regioselectivity: -
Sterioselectivity: Anti Addition
Intermediate: Bromonium Ion
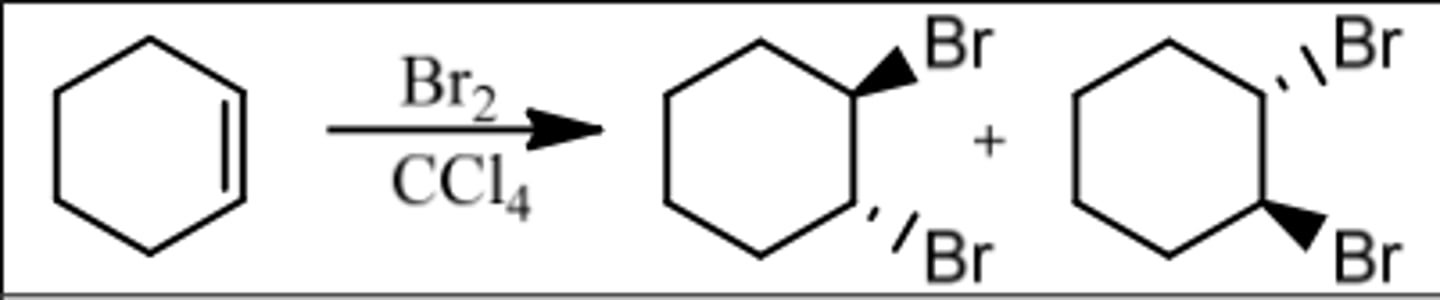
Reaction: Bromination in H₂O
Reagents: Br₂/H₂O (or Cl₂/H₂O)
What's Added: Br⁺ & OH⁻ (or Cl⁺ & OH⁻)
Regioselectivity: Markovnikov
Sterioselectivity: Anti Addition
Intermediate: Bromonium Ion
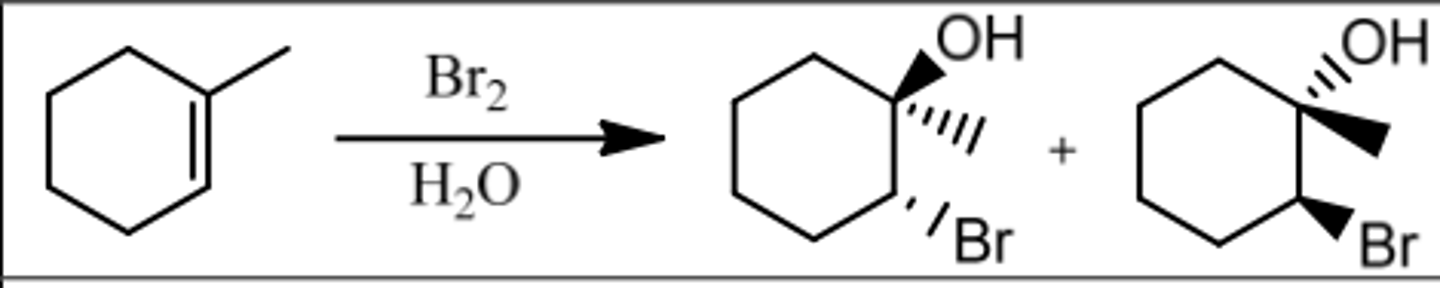
Reaction: Bromination in Alcohol
Reagents: Br₂/ROH (Cl₂/ROH)
What's Added: Br⁺ & OR⁻ (or Cl⁺ & OR⁻)
Regioselectivity: Markovnikov
Sterioselectivity: Anti Addition
Intermediate: Bromonium Ion
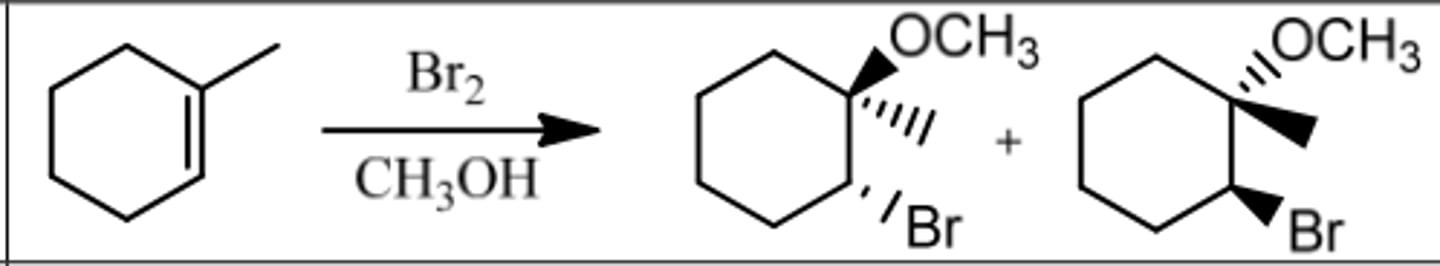
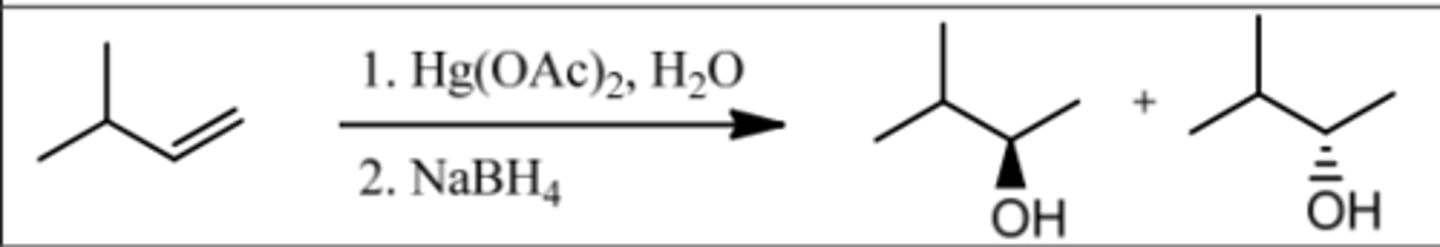
Reaction: Oxymercuration-Demurcuration
Reagents: 1. Hg(OAc)₂, H₂O
2. NaBH₄
What's Added: H⁺ & OH⁻
Regioselectivity: Markovnikov
Sterioselectivity: Anti Addition
Intermediate: Mercurinium Ion
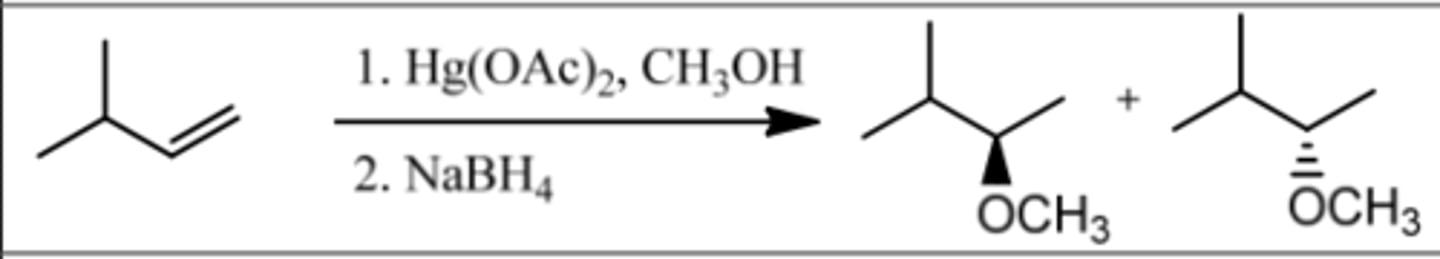
Reaction: Alkoxymercuration-Demercuration
Reagents: 1. Hg(OAc)₂, ROH
2. NaBH₄
What's Added: H⁺ & OR⁻
Regioselectivity: Markovnikov
Sterioselectivity: Anti Addition
Intermediate: Mercurinium Ion
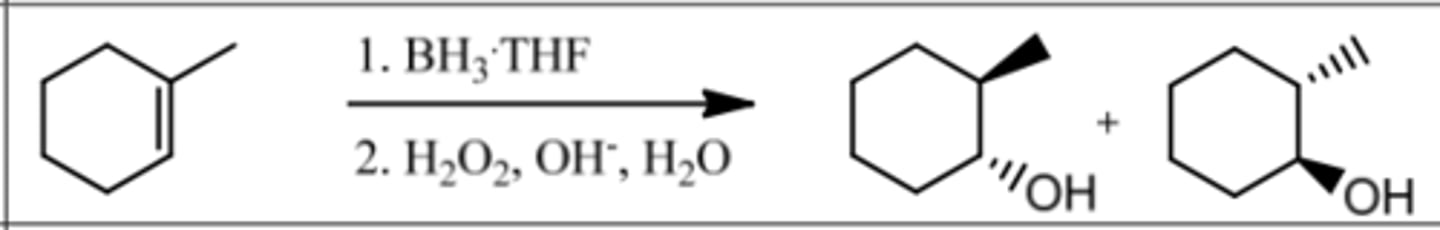
Reaction: Hydroboration-Oxidation
Reagents: 1. BH₃∙THF
2. H₂O₂, OH⁻, H₂O
What's Added: H⁺ & OH⁻
Regioselectivity: Anti-Markovnikov
Sterioselectivity: Syn Addition (H⁺ & OH⁻ are Syn)
Intermediate: -
Reaction: Catalytic Hydrogenation (Catalytic Reduction)
Reagents: H₂/Catalyst
(Catalyst = Pt/C, Pd/C, or Ni)
What's Added: H & H
Regioselectivity: -
Sterioselectivity: Syn Addition
Intermediate: -
[Forms Meso Compound]
![<p>Reagents: H₂/Catalyst</p><p>(Catalyst = Pt/C, Pd/C, or Ni)</p><p>What's Added: H & H</p><p>Regioselectivity: -</p><p>Sterioselectivity: Syn Addition</p><p>Intermediate: -</p><p>[Forms Meso Compound]</p>](https://knowt-user-attachments.s3.amazonaws.com/3028baa7-7a22-4459-8693-754609112ff8.jpg)
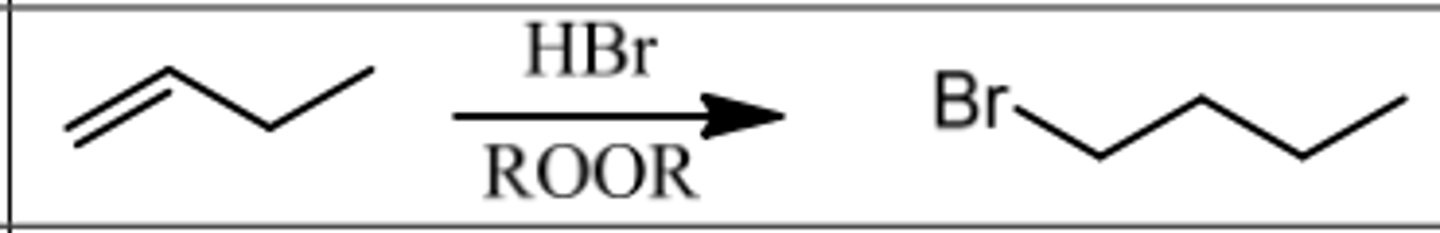
Reaction: Hydrobromination w/ Peroxide
Reagents: HBr/ROOR (Peroxide)
What's Added: H∙ & Br∙ Regioselectivity: Anti-Markovnikov
Sterioselectivity: -
Intermediate: Radical
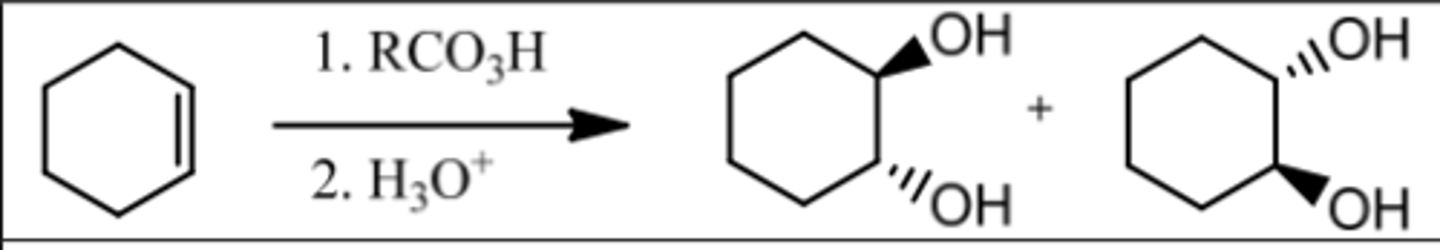
Reaction: Anti-Hydroxylation
Reagents: 1. RCO₃H (MCPBA)
2. H₃O⁺
What's Added: OH & OH
Regioselectivity: -
Sterioselectivity: Anti Addition
Intermediate: Epoxide
Reaction: Epoxidation
Reagents: 1. RCO₃H (or MCPBA)
What's Added: OH & OH
Regioselectivity: -
Sterioselectivity: Anti Addition
Intermediate: Epoxide
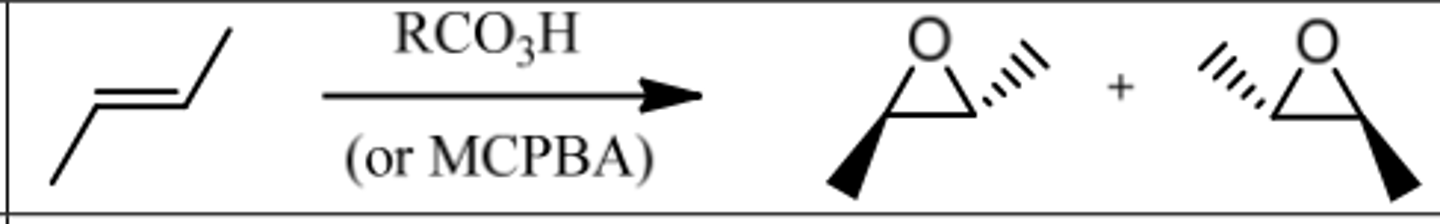
Reaction: Syn-Hydroxylation
Reagents: 1. OsO₄
2. H₂O₂
What's Added: OH & OH
Regioselectivity: -
Sterioselectivity: Syn Addition
Intermediate: -
[Forms Meso Compound]
![<p>Reagents: 1. OsO₄</p><p>2. H₂O₂</p><p>What's Added: OH & OH</p><p>Regioselectivity: -</p><p>Sterioselectivity: Syn Addition</p><p>Intermediate: -</p><p>[Forms Meso Compound]</p>](https://knowt-user-attachments.s3.amazonaws.com/76ed51ea-adb9-4062-a774-aeee85dea251.jpg)
Reaction: Syn Hydroxylation
Reagents: KMnO₄ (cold, dilute)/ OH⁻
What's Added: OH & OH
Regioselectivity: -
Sterioselectivity: Syn Addition
Intermediate: -
[Forms Meso Compound]
![<p>Reagents: KMnO₄ (cold, dilute)/ OH⁻</p><p>What's Added: OH & OH</p><p>Regioselectivity: -</p><p>Sterioselectivity: Syn Addition</p><p>Intermediate: -</p><p>[Forms Meso Compound]</p>](https://knowt-user-attachments.s3.amazonaws.com/c252fa84-1f02-49db-ad2f-d22b5cd45ac2.jpg)
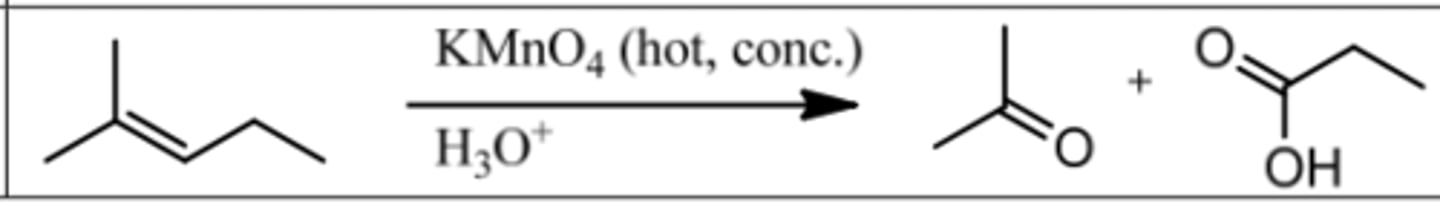
Reaction: Oxidative Clevage
Reagents: KMnO₄ (hot, conc.)/ H₃O⁺
What's Added: Cleave double bond and add OH to less substituted C.
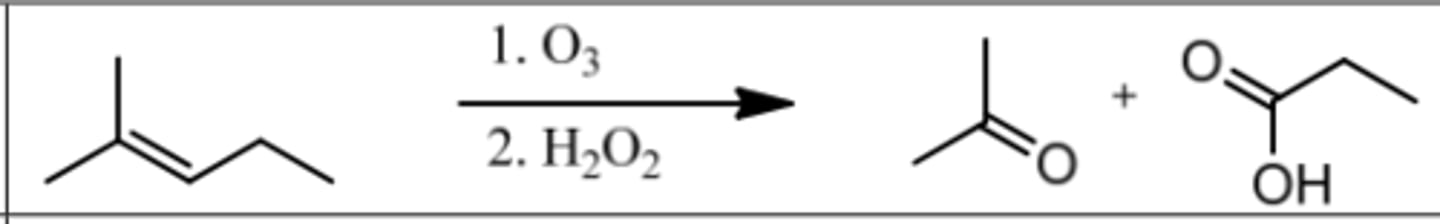
Reaction: Ozonolysis (under Oxidizing Conditions)
Reagents: 1. O₃
2. H₂O₂ (Peroxide)
What's Added: Cleave double bond and add OH to less substituted C.
Reaction: Ozonolysis (Under Reducing Conditions)
Reagents: 1. O₃
2. (CH₃)₂S or (Zn/H₂O)
What's Added: Cleave double bond and add H (Aldehyde) to less substituted C