Immunology 15 | Lymphocyte Development
1/21
Earn XP
Description and Tags
Helpful video: https://www.youtube.com/watch?v=w4GXsoow2SM; https://www.youtube.com/watch?v=1JpzY1oEDsw
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
What is a double positive T Cell
A double-positive T cell is an immature T cell in the thymus that expresses both CD3, CD4 and CD8 surface markers, along with CD3. This stage represents an intermediate phase in T cell development, where T cells undergo selection.
What is a double negative T Cell
A double-negative T cell is an early-stage immature T cell in the thymus that expresses CD3 but lacks both CD4 and CD8 surface markers. This stage occurs before the T cell commits to either the CD4+ helper T cell or CD8+ cytotoxic T cell lineage during its development.
Where does lymphocyte maturation begin?
In the bone marrow with hematopoietic stem cells
Where do B lymphocytes and T lymphocytes mature?
B lymphocytes mature in the secondary lymphoid organs
T lymphocytes mature in the thymus
Positive and negative selection of lymphocytes is based upon
antigen receptor expression
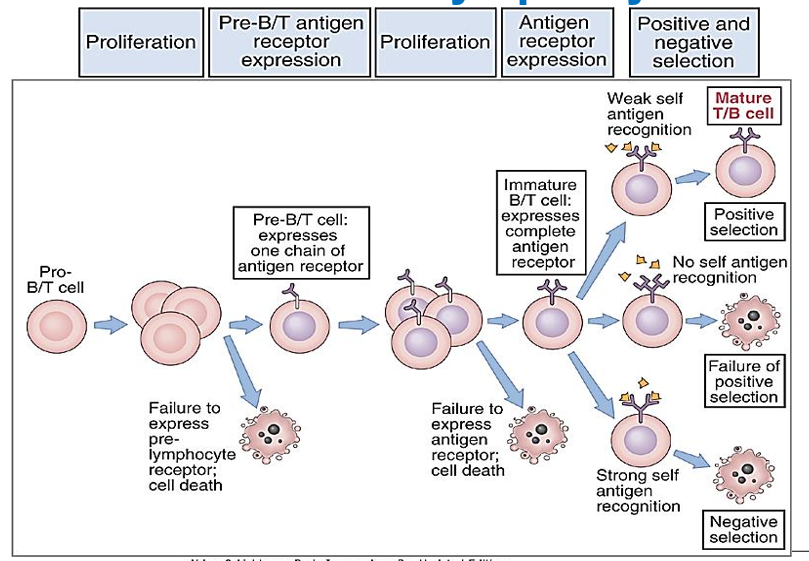
Describe the concept of positive selection in T Cells
T cells are tested to see if their receptors can recognize and bind weakly to self-MHC molecules in the cortex of the thymus.
Double-positive (CD4+8+) T cells bind to cortical thymic epithelial cells (cTECs) in the thymus. These cTECs present self-MHC molecules with self-peptides. T cells that can moderately bind to these MHC molecules receive survival signals and are positively selected to continue maturing.
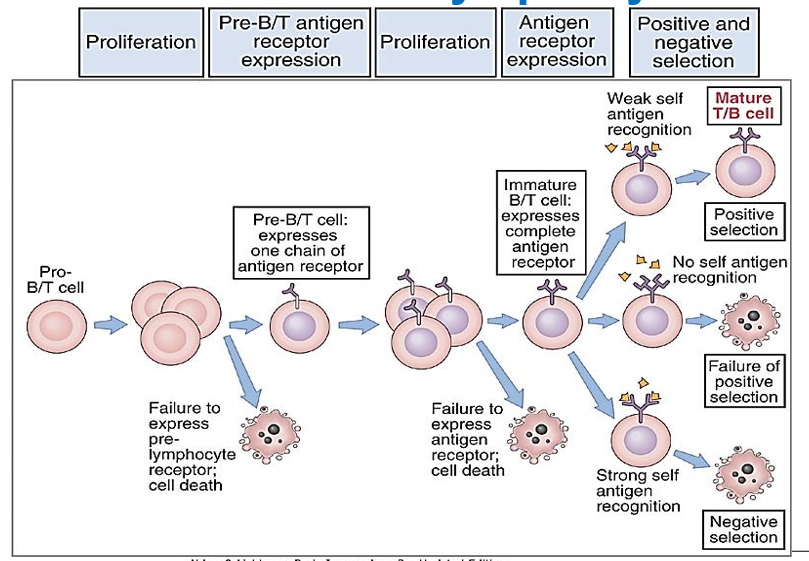
Describe the concept of negative selection in T Cells
T cells that bind too tightly to self-antigens presented by self-MHC molecules are eliminated in the medulla of the thymus. “Death by neglect”.
T cells that survive positive selection move to the medulla of the thymus, where they encounter medullary thymic epithelial cells (mTECs), as well as other antigen-presenting cells like dendritic cells. Here, T cells that bind too strongly to self-antigens presented by self-MHC molecules are eliminated through negative selection, preventing autoimmunity.
How are Tregs selected in the thymus?
Tregs undergo positive selection. They are chosen based on having the highest levels of "acceptable" affinity for binding peptides with their T cell receptors, meaning they can recognize self-antigens but not too strongly.
They bind strong but not too strongly and are within the threshold of being selected for positive selection. This is called High Avidity
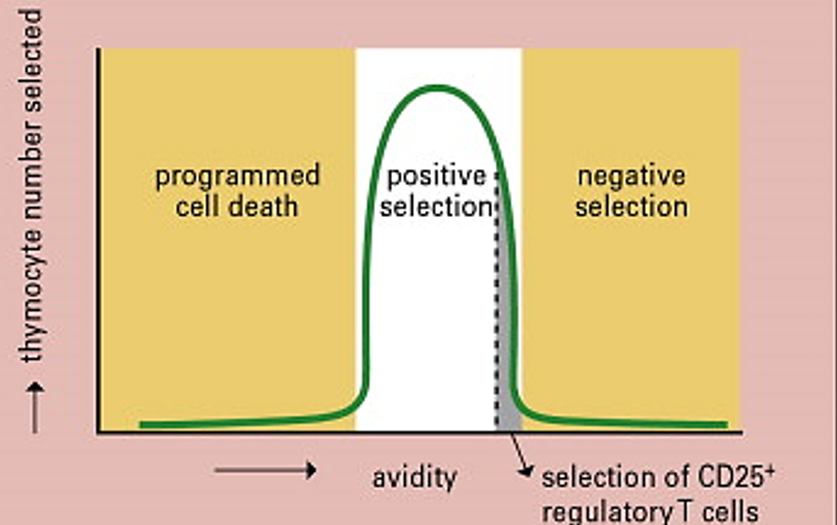
Double-negative cells develop in which part of the thymus?
Cortex of thymus
Note: DN and DP development are different than positive and negative selection.
Double-positive cells develop in which part of the thymus?
Medulla of the thymus
Note: DN and DP development are different than positive and negative selection.
Describe the T Cell Maturation process step by step
Early Stage: T cell progenitors (young T cells) in the thymus start off as "double-negative" (DN) cells, meaning they don't yet express CD4 or CD8 surface markers. They do express CD2 and CD3, which are early markers of T cell development.
Receptor Formation: The genes that make up the T cell receptor (TCR) rearrange, creating a "rough draft" TCR that can potentially recognize antigens. This involves recombining VDJ gene segments to form the two TCR chains (α and β).
Double Positive Stage: The developing T cells begin to express both CD4 and CD8 markers, becoming "double-positive" cells.
Selection Processes:
Positive Selection: T cells that can properly bind to self-MHC molecules on thymic cells survive.
Negative Selection: T cells that do not react too strongly to self-antigens survive. Those that fail either selection die by apoptosis.
Single Positive Stage: Depending on whether the TCR binds better to MHC Class I or II, the cell will express either CD8 (for Class I) or CD4 (for Class II) and become "single-positive".
Maturation: Mature, but naïve, T cells that pass both selections are released into the bloodstream. However, about 99% of T cell progenitors do not survive the selection process in the thymus.
What are the two defining markers for Tregs?
CD25 and FoxP3
Are Tregs found and low levels or high levels in blood?
low levels.
Tregs are found at low levels in the blood because they primarily function in tissues and lymphoid organs, where they regulate and suppress local immune responses. Their presence in low levels helps maintain a balance, preventing autoimmunity without compromising the body's ability to fight infections.
Which T cells recognize lipid antigens and stress proteins?
gamma/delta (γδ T cells)
Which T cells recognize glycolipid antigens?
Natural Killer T Cells
What are the two B cell lineage-specific markers?
CD19 and CD20 are two B cell lineage-specific markers.
Describe the B Cell Checkpoints step by step
Heavy Chain Checkpoint: The B cell first checks if a functional heavy chain is made by pairing it with a surrogate light chain to form a "pre-BCR." If successful, the cell proceeds to rearrange the light chain genes. If not, the cell undergoes apoptosis.
Light Chain Checkpoint: Next, the cell checks if a functional light chain is produced. If successful, the B cell can exit the bone marrow. If the initial attempt fails, the cell has a second chance by rearranging a different light chain locus (κ or λ). If this fails again, the cell dies.
Occurs in the bone marrow
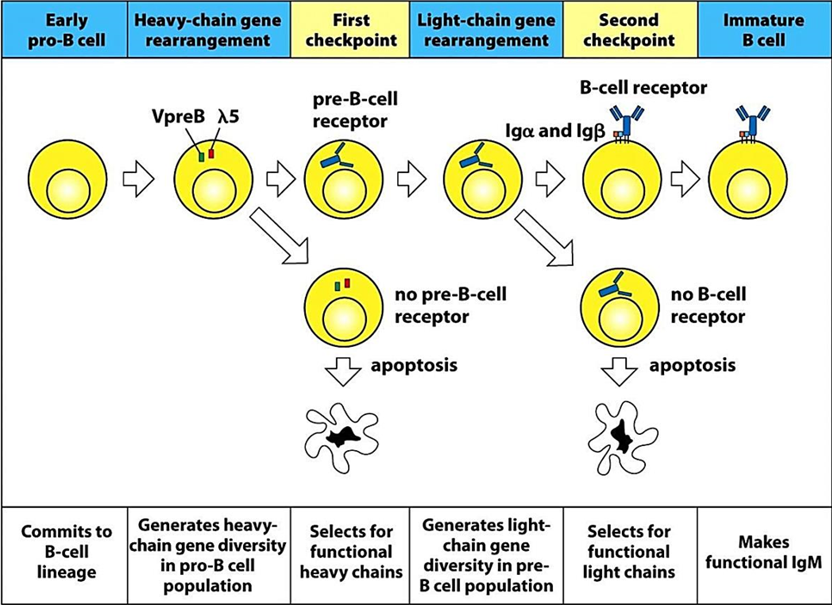
Where do the B Cell checkpoints occur?
In the bone marrow
What happens when the B cell enters the lymph node and encounters the correct antigen?
It undergoes two more changes to optimize its effector function
1. Affinity Maturation: the process by which B cells improve the binding strength of their receptors to a specific antigen through repeated mutations and selection
2. Isotype switching: When a B cell changes the type of antibody it produces (like IgM to IgG, IgA, or IgE) to better suit the body's defense needs against a particular pathogen.
How are B Cell Receptors refined after antigen exposure?
When a B cell binds to a specific antigen, it begins to divide, and its receptors hypermutate with each new generation of cells. This process, called affinity maturation, helps the B cell improve its ability to recognize the antigen: cells with weaker binding are eliminated, while those with stronger binding survive and continue to mutate. Eventually, the B cell matures into a plasma cell, changes the type of antibody it produces (isotype switching), and makes antibodies like IgG, IgE, or IgA that are best suited to fight the infection.
What is the ultimate goal of lymphocyte development?
Generation of sufficient numbers T and B cells with sufficient diversity in antigenic specificity
What is allelic exclusion in B cells?
Allelic exclusion in B cells ensures that each B cell produces a unique antibody by rearranging and expressing only one allele of the immunoglobulin gene at a time, preventing dual expression of different antibody specificities.