Activation Energy Diagram
0.0(0)
Card Sorting
1/5
Earn XP
Description and Tags
Last updated 1:40 AM on 4/29/24
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
1
New cards
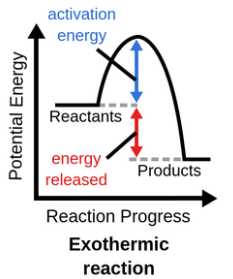
Exothermic Reaction
Products have less energy than reactants
2
New cards
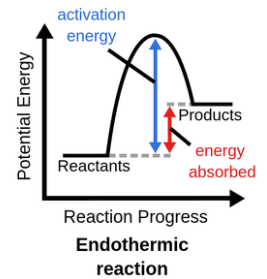
Endothermic Reaction
Products have more energy than reactants
3
New cards
Reactants
Chemicals put into reaction and at very left of graph
4
New cards
Activated Complex
Highest point; highest energy and most unstable; bonds not broken or formed
5
New cards
Products
Output of reaction; Far right of graph
6
New cards
Catalyst
Results it lower peak due to it introducing a path of lower activation energy