IUPAC
1/50
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
Who said that organic shit can be made from inorganic ; What chemical did he use and what was the result that he got
Kolbe; He used Ammonia cyanate, subjected this to heat, and created urea
Write the electronegativity series for F, O , N and C (at different hybridization states)

What are super primary carbons
The carbon atoms that aren’t attached to any other carbon; The carbon atom having 0 degrees of carbon in it
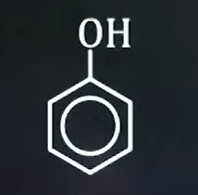
What is the bond line structure for phenol
What group in the periodic table contains the halogens?
The elements that present in Group 7 are called halogens
List all of the elements that are present in group 7 of the periodic table (Top to bottom)
Fluorine, Chlorine, Bromine, Iodine, Astatine, Tennessine
What is the degree of unsaturation
It is the number of pi bonds/rings present in a compound
What are the different terms that all refer to the degree of unsaturation

What formula do we use when we want to calculate the Degree of unsaturation when we are given Molecular formula
D.U = 2C+2+N−H−X/2; Where,
C = number of carbon atoms
H = number of hydrogen atoms
N = number of nitrogen atoms
X = number of halogens (F, Cl, Br, I)
What are homocyclic compounds
It is a cyclic compound in which only C atoms are present
What is the rule for writing IUPAC for a given compound?
Prefix 2 + Prefix 1 + Root Word + Suffix 1 + Prefix 2 +
What does prefix 2 contain
It contains
1. Halo → X (F, Cl, Br, I…)
Alkyl → R — (some compound)
Alkoxy → OR — (some compound)
Nitro → NO₂ — (some compound)
Nitroso → NO — (some compound)
What does the ‘R’ mean in Prefix 2 when naming compounds? What is its general formulae
R is referring to the Alkyl group
R is a wildcard placeholder for any sub-carbon chain.
Its general formula is Cn+H2n+1
What does prefix 1 contain
It contains cyclo compounds (if any)
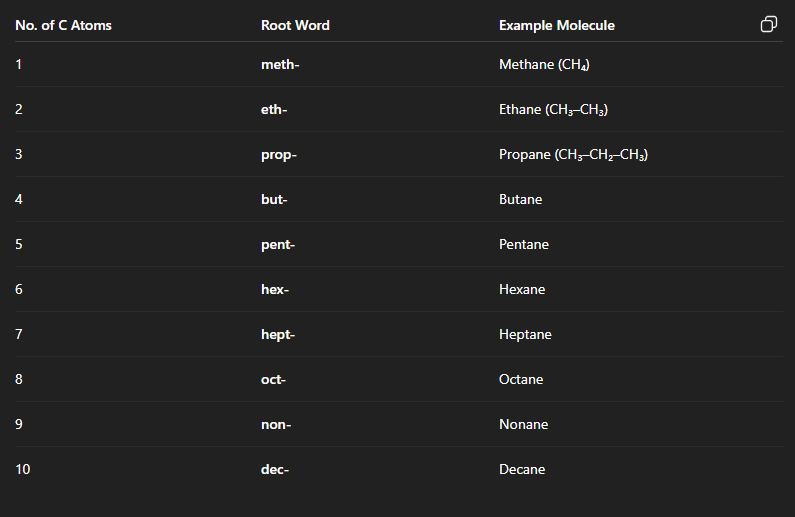
What does the Root word/ Principal chain have? Name them from 1-10
It has the number of Carbon atoms present in a single chain
What does suffix 1 contain
C—C 1 bond → ane
C—C 2 bond → ene
C—C 3 bond → yne
What does suffix 2 contain
Names of the functional groups that are attached to the parent chain
What is the 1st priority when numbering a compound?
singular PFG
What is the 2nd priority when numbering a compound?
Multiple/Double Bonds
What is the 3rd priority when numbering a compound?
Longest Chain of C atoms
What is the 4th priority when numbering a compound?
Maximum number of substituents
What is the 5th priority when numbering a compound?
Lowest Locant for a PFG
What is the 6th priority when numbering a compound?
Lowest Locant for a Multiple Bond
What is the 7th priority when numbering a compound?
Lowest Locant for a prefix
After all of the numbering is done, how would you write the compound
Alphabetical Order, ignoring the di, tri, etc
What gets the 1st priority when writing a suffix-2 (PFG) and prefix -2; What is its Formula?; What will be its special suffix when it’s connected to a closed cyclic compound
Carboxylic acid
oic acid (suffix-2)
Carboxy (prefix -2)
COOH
Carboxylic acid
What gets the 2nd priority when writing a suffix-2 and prefix -2 (PFG); What is its Formula? ; What will be its special suffix when it’s connected to a closed cyclic compound
Sulphonic acid
Sulphonic acid (suffix-2 )
Sulpho (prefix -2 )
SO3H
Sulphonic acid (same as suffix 2)
What gets the 3rd priority when writing a suffix-2 and prefix -2 (PFG); What is its Formula? ; What will be its special suffix when it’s connected to a closed cyclic compound
Anhydride
oic anhydride (suffix-2)
- (prefix -2 )

What gets the 4th priority when writing a suffix-2 and prefix -2 (PFG); What is its Formula? ; What will be its special suffix when it’s connected to a closed cyclic compound
Ester
oate (suffix-2)
Alkoxycarbonyl (prefix -2 )
carboxylate (special suffix)
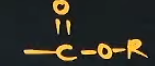
What gets the 5th priority when writing a suffix-2 and prefix -2 (PFG); What is its Formula? ; What will be its special suffix when it’s connected to a closed cyclic compound
Acid Chloride
oyl chloride (suffix-2)
chlorocarbonyl (prefix -2)
carbonyl chloride (special suffix)

What gets the 6th priority when writing a suffix-2 and prefix -2 (PFG); What is its Formula? ; What will be its special suffix when it’s connected to a closed cyclic compound
Acid amide
amide (suffix-2)
carbamoyl (prefix -2 )
carboxamide (special suffix)

What gets the 7th priority when writing a suffix-2 and prefix -2 (PFG); What is its Formula? ; What will be its special suffix when it’s connected to a closed cyclic compound
Cyanide
nitrile (suffix-2)
cyano (prefix -2 )
—CN
carbonitrile (special suffix)
What gets the 8th priority when writing a suffix-2 and prefix -2 (PFG); What is its Formula? ; What will be its special suffix when it’s connected to a closed cyclic compound
Iso Cyanide
iso nitrile (suffix-2)
isocyano (prefix -2 )
—NC
-
What gets the 9th priority when writing a suffix-2 and prefix -2 (PFG); What is its Formula? ; What will be its special suffix when it’s connected to a closed cyclic compound
Aldehyde
al (suffix-2)
aldo and formyl (prefix -2 )
CHO
carbaldehyde (special suffix)
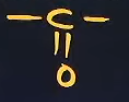
What gets the 10th priority when writing a suffix-2 and prefix -2 (PFG); What is its Formula? ; What will be its special suffix when it’s connected to a closed cyclic compound
Ketone
one (suffix-2)
keto or oxo (prefix -2 )
one (same as suffix-2)
What gets the 11th priority when writing a suffix-2 and prefix -2 (PFG); What is its Formula? ; What will be its special suffix when it’s connected to a closed cyclic compound
Alcohol
ol (suffix-2)
hydroxy (prefix -2 )
OH
ol (same as suffix-2)
What gets the 12th priority when writing a suffix-2 and prefix -2 (PFG); What is its Formula? ; What will be its special suffix when it’s connected to a closed cyclic compound
Thiol
thiol (suffix-2)
mercapto (prefix -2 )
—SH
thiol (same as suffix-2)
What gets the 13th priority when writing a suffix-2 and prefix -2 (PFG); What is its Formula? ; What will be its special suffix when it’s connected to a closed cyclic compound
amine
amine (suffix-2)
amino (prefix -2)
—NH2
amine (same as suffix-2)

What is this used to represent?
It is used to represent a bond that is coming out of the plane like an aeroplane coming at you (literally towards you)

What is this used to represent?
It is used to represent a bond that is going into the plane like a missile that you sent back cause a plane was coming at your face (literally towards you)
What do we name the prefix of a benzene ring?
We name it phenyl
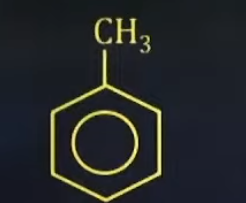
State the common name of this compound
Toulene

Name the common name for all 2 of these compounds
Phenol
Benzaldihyde
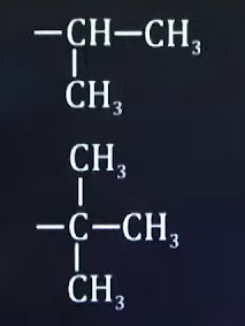
What are the common names of these sub-sub-branches
iso
tert
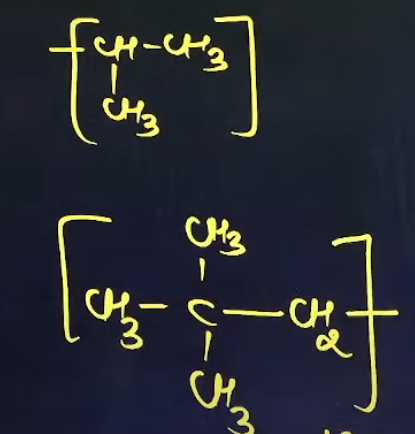
What are the common names of these sub-sub-branches
iso
neo
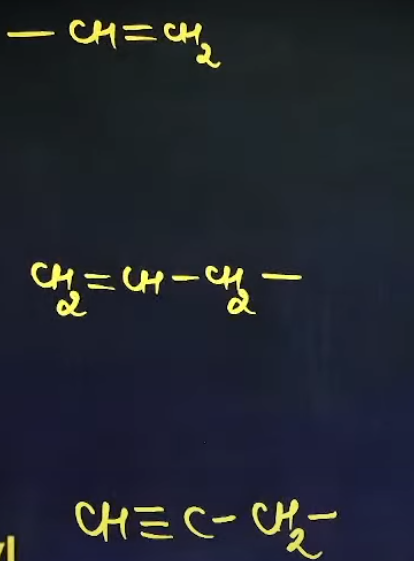
What are the common names of these sub-sub-branches
Vinyl
Allyl
Propargyl
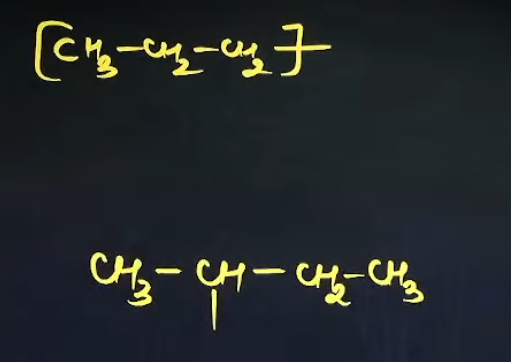
What are the common names of these sub-sub-branches
n
sec

What are the common names of these sub-sub-branches
gem
vic
Alkylidene
Note :- gem and Alkylidene are the same
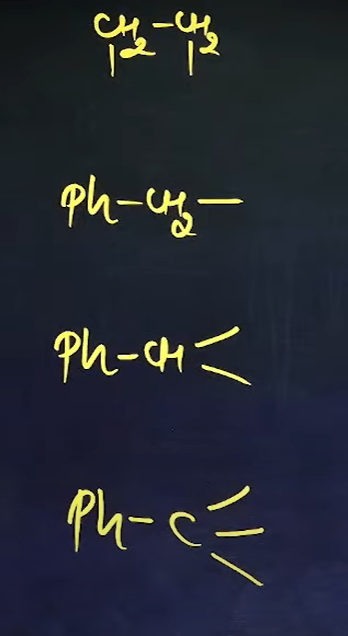
What are the common names of these sub-sub-branches
Alkylene
Benzyl
Benzal
Benzo

What do you call this?
Phenyl
What group has the prefix of ‘oxy’; What is its formulae?
“‑oxy” is the prefix for an alkoxy group, which is basically an ether substituent (–O–R).
Example:
– Methoxy‑ = –O–CH₃
– Ethoxy‑ = –O–CH₂CH₃