pchem quizzes
1/300
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
301 Terms
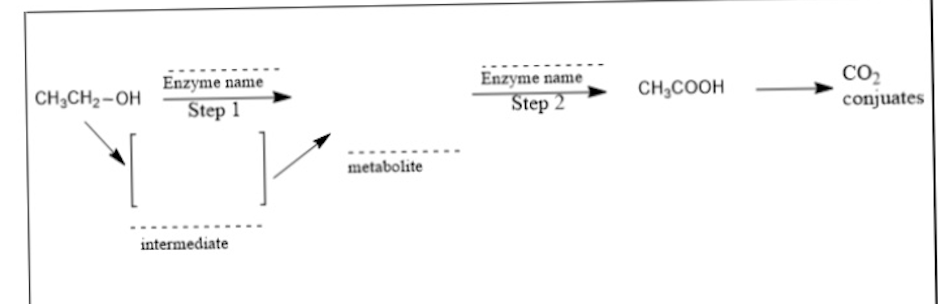
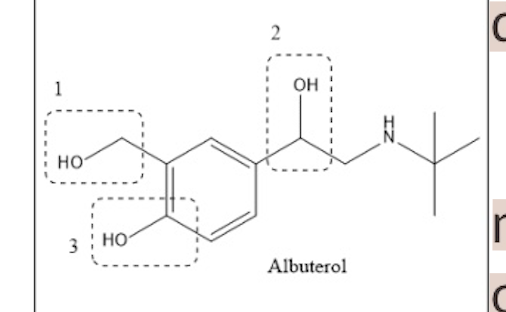
What is the name of the metabolite in the middle with the dotted line?
acetaldehyde
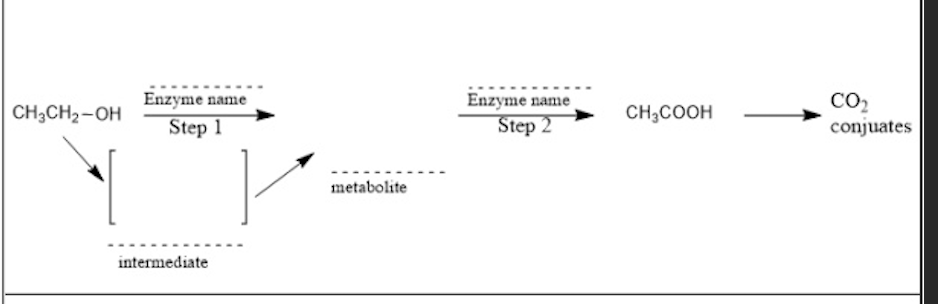
Which of the following enzymes are involved in the step 2 of its metabolism?
b. aldehyde dehydrogenase
c. aldehyde oxidase
e. xanthine oxidase
The most important site of drug metabolism is the
liver
Which of the following statements is/are true about serotonin?
I. Serotonin is a monoamine neurotransmitter in the CNS.
II. Serotonin is not oxidatively deaminated in humans.
III. All serotonin receptors are GPCRs.
I only
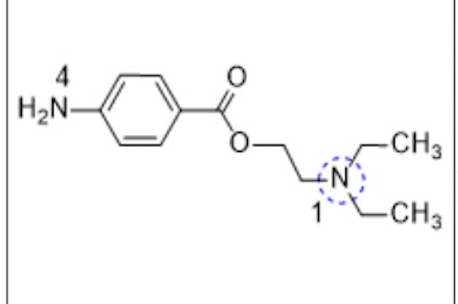
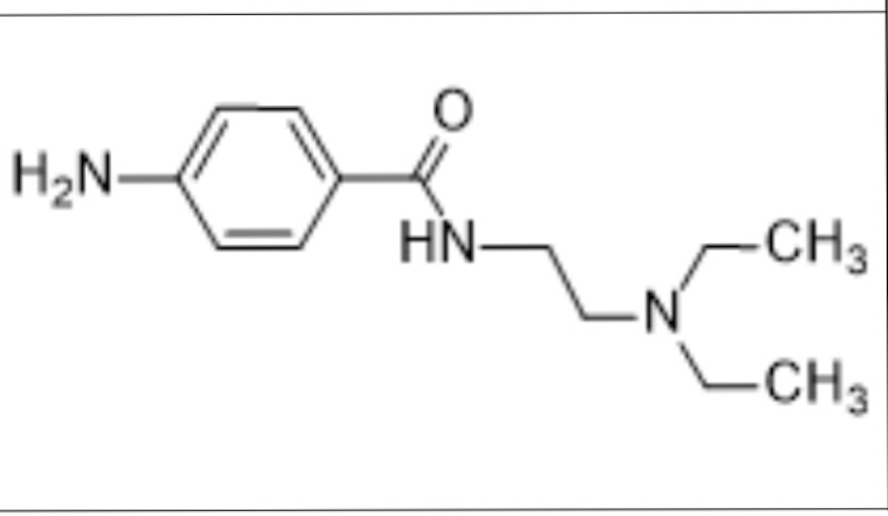
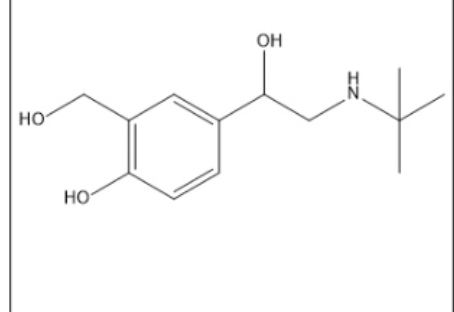
The drug illustrated below:
I. can undergo metabolically oxidative N-dealkylation.
Il. can undergo metabolically oxidative deamination at the N marked as 1
Ill. can undergo metabolically oxidative deamination at the 4-NH2.
I, II, and III
True or False: All phase I metabolism is followed by a phase Il metabolism.
False
Which of the following is/are substrates of glutathione S-transferase?
a. Alkyl halides
b. Aryl halides
c. Epoxide
e. a, B-unsaturated carbonyl compounds
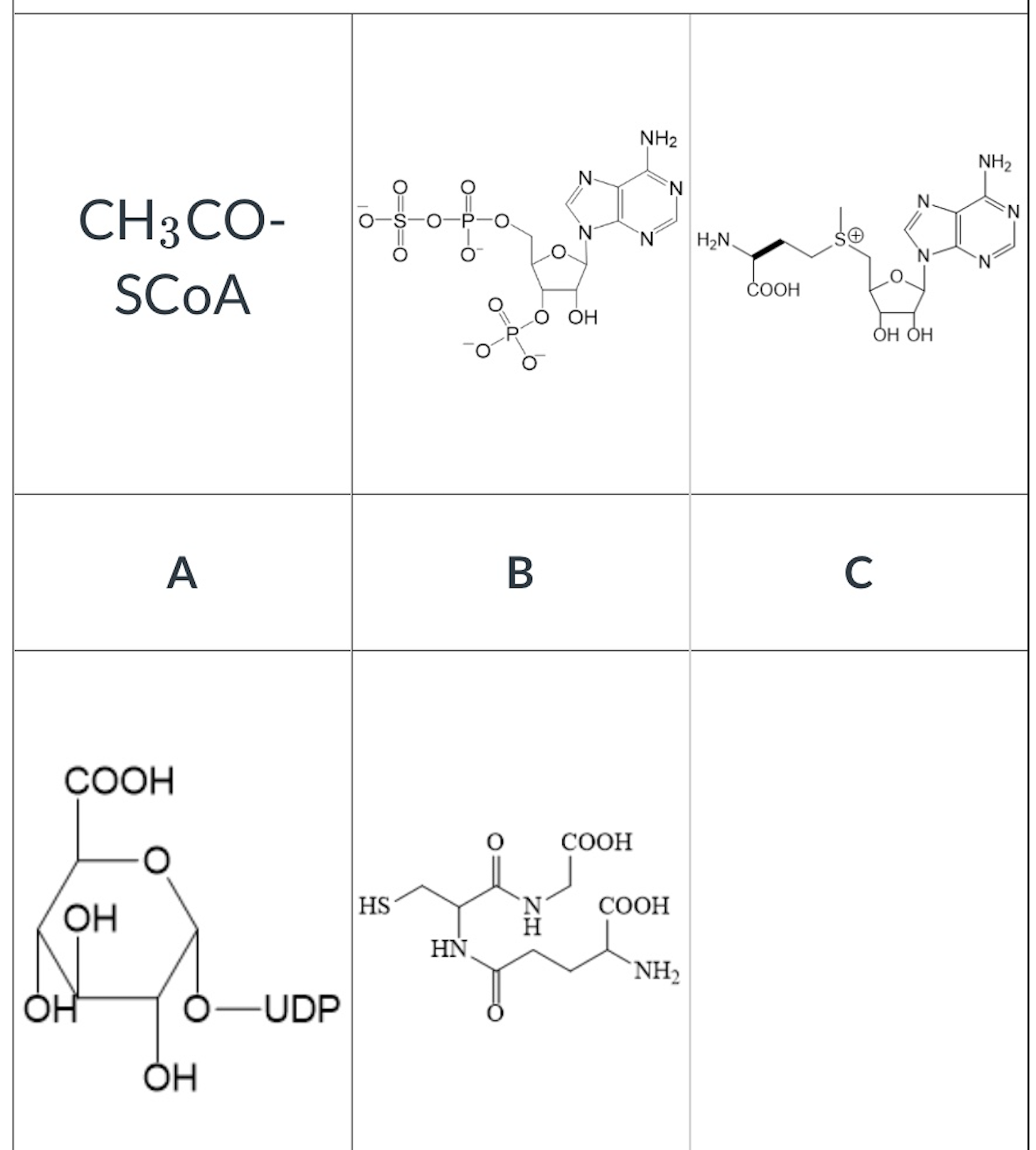
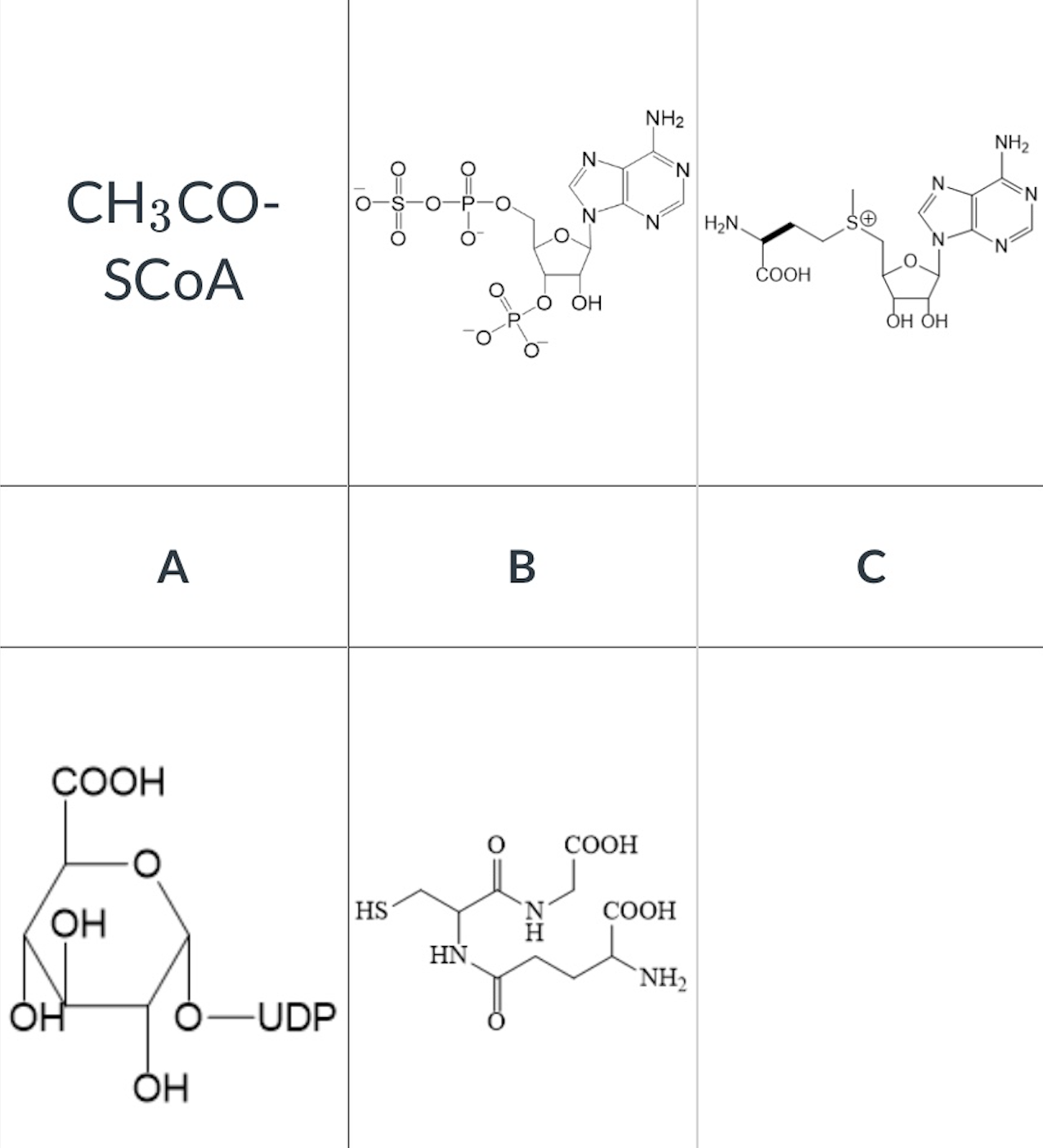
which of the following is the cosubstrate of COMT?
C
The drug illustrated below can undergo the following phase II reactions:
a. acetylation
b. glucuronidation
Select All That Apply:
Which of the following functional groups can undergo sulfation reactions metabolically in vivo?
c. Aromatic 1* -amine
e. phenol
Which of the following is the cosubstrate for glucuronidation reaction?
a. amides
c. phenols
d. tertiary amines
Gel electrophoreses is used to:
a. Separate macromolecules based on molecular weight
b. Separate macromolecules based on charge
c. Both (A) and (B)
d. Neither (A) nor (B)
D
True or False: The purpose of a guard column is to protect the main column.
True
True or False: Peak resolution in chromatography should be at least 1.15.
False
True or False: Diffusion in liquids is faster than diffusion in a gas.
False
True or False: The main variables in GC are eluant and column.
False
True or False: On reversed phase HPLC using a C18 column, it is the hydrophobic end that interacts with the stationary phase.
True
True or False: An ultraviolet detector is useful with a gradient.
True
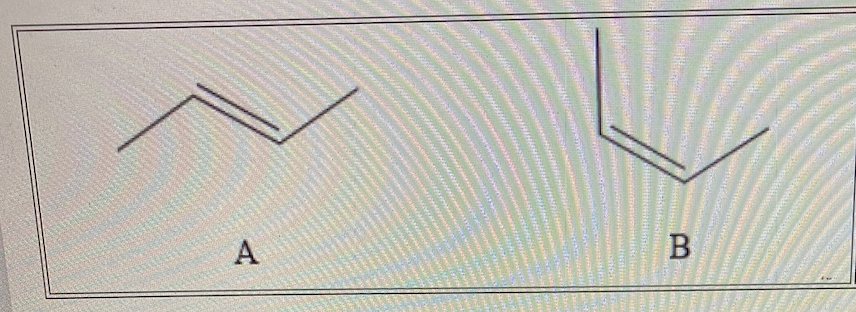
Consider the two structures A and B below. This is a pair of what type(s) of isomers?
I. Cis-trans isomers
II. Diastereomers
III. Enantiomers
I and II only
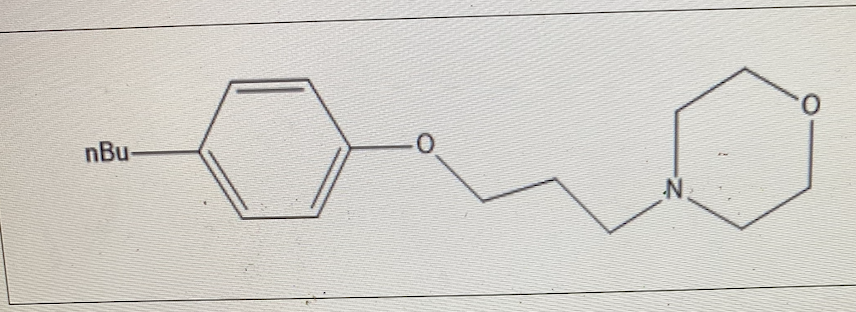
For the topical analgesic Pramoxine, the pKa was determined to be 7.
In the bloodstream, what is the % ionization?
50%
Which of the following methods is most useful in identification of certain functional groups that contain triple bonds?
IR
Which of the following chemicals is most unlikely to be used as a medicine to treat or prevent a disease?
sodium hydroxide
Which of the following functional groups is acidic?
-COOH
Which of the following properties is unlikely to affect a drug's behavior in the human body?
melting point
What would be the net charge of glutamic acid at pH 7?
negative
True or False: Most drugs are organic compounds.
true
Select All That Apply. Which of the following elements are least likely to be present in organic drug molecules?
b. helium
d. lead
Which of the following functional groups is basic?
-CH2NH2
Mark the Best Answer. Which of the following organic compounds is/are found in the human body?
all of the above
(carbs, lipids, nucleic acids, proteins)
The drug below can
I. undergo metabolically oxidative N-dealkylation.
Il. undergo metabolically oxidative deamination.
Ill. be oxidized metabolically at alcohol 1 to an acid.
II and III only
Which of the following statements is/are true about dopamine?
I. Dopamine carries one positive charge at physiological pH.
II. Dopamine is a substrate of catechol O-methyl transferase (COMT).
Ill. Dopamine can easily cross the blood-brain barrier.
I and II only
Which of the following statements is/are true about
GABA?
I. GABA is zwitterionic and charge neutral under physiological pH.
II. GABA receptors are all GPCRs
IlI. GABA relies on metabolism for its elimination from the synapse.
I only
Which of the following statements is/are true about histamine?
I. All histamine receptors are GPCRs.
II. It has two basic functional groups.
III. The predominant form under physiological pH is histamine with two positive charges.
I and II only
Which of the following functional groups can be metabolically reduced in vivo?
a. amide
b. aromatic nitro group
e. ketone
The drug below can
I. I. be sulfated metabolically.
II. be glucuronidated metabolically.
Ill. be methylated metabolically by COMT.
I and II only
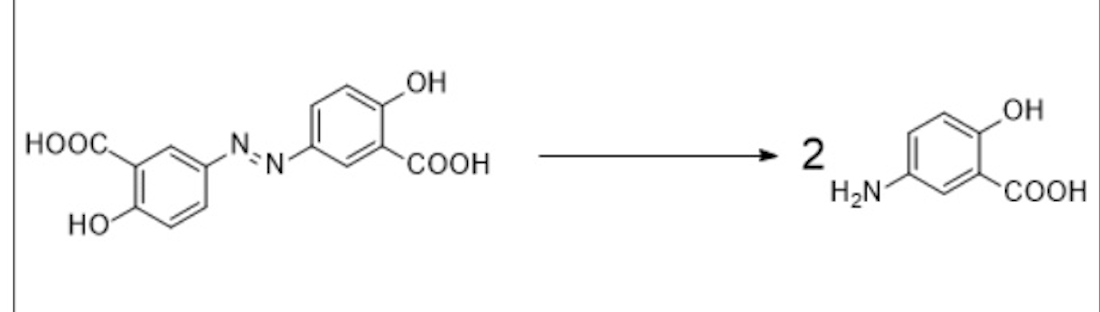
What is the enzyme involved in the following metabolic conversion?
Bacterial azo reductase
Which of the following is a substrate for conjugation with amino acids in vivo?
Carboxylic acid
Which of the following statements about chromatography are true?
I. Separates a mixture into its components
II. Can use a packed column, an open tubular column or a thin-coated sheet
III. Mobile phases can be gas, liquid or solid
IV. Stationary phases can be liquid or solid
I, II, and IV only
True or False: A cation exchange column uses a solid phase with negatively charged groups.
True
True or False: An analyte with a high affinity for the mobile phase will elute slower than one with a high affinity for the stationary phase.
False
True or False: To extract a base from the aqueous phase to the organic phase, the pH of the aqueous phase needs to be acidic.
false
The mechanism by which partition chromatography separates compounds is:
Dissolution into a liquid phase
A metal ion can be removed from the aqueous phase by using a chelating ligand and appropriate adjustment of the pH of the aqueous layer.
true
In a chromatographic separation:
I. A solute which has low affinity for the packing material will elute more slowly than one which has a strong affinity
Il. Differential migration contributes to the separation
III. The Gaussian distribution is not affected by band broadening
IV. An analyte which elutes quickly will be sharper than one which elutes slowly
II and IV only
True or False: The peak asymmetry factor, AF, should be in the range of 0.90 to 1.15.
false
In an open tubular column, the A term is zero because:
There is no packing material
Select All That Apply:
Decreasing particle size results in which of the following?
b. decreasing plate height
c. increasing column pressue
d. better resolution
e. increasing plate number
Select All That Apply:
Which of the following are causes of peak fronting on HPLC?
b. overloading
c. poor trapping of the solute by the stationary phase
e. injection solvent too strong
Which of the following factors affect resolution?
Stationary phase
Retention time
Capacity factor
Column length
Column efficiency
True or False: The van Deemter equation includes parameters for eddy diffusion, column efficiency, longitudinal diffusion, resistance to mass transfer and linear velocity.
false
True or False: Diffusion in liquids is faster than diffusion in a gas.
false
True or False: A mass spectrometer has low selectivity.
false
Select All That Apply:
Which of the following statements) are correct about an HILIC column?
b. Uses a hydrophilic stationary phase
e. The stationary phase is zwitterionic
Select All That Apply:
Which of the following correctly completes the sentence "An ideal detector needs to...'“?
a. be sensitive
d. be selective
e. be universal
Select All That Apply:
Which of the following factors affect retention and selectivity in reversed phase HPLC?
mobile phase
stationary phase
temperature
pH
eluant flow rate
which of the following statement(s) are correct Normal Phase
Chromatography?
d. more polar compounds will elute before less-polar compounds
e. can use cyano or diol bonded phases
Which of the following statements) are correct about Reverse Phase Chromatography?
b. Uses water as part of the mobile phase
d. more polar compounds will elute before less-polar compounds
e. can use cyano or diol bonded phases
True or False: An external standard is used to provide correction for loss during sample preparation.
False
Select All That Apply:
Which of the following statements) are correct about a gradient elution in liquid chromatography?
a. On normal phase starts with the least polar solvent and ends with the most polar solvent
c. On HILIC starts with the least polar solvent and ends with the most polar solvent
d. Moves strongly retained components of the mixture faster while enabling recalution of the least retained
e. can use ion pairing agents
All clinical trials of a potential new drug are done in patients with the intended disease.
false
How many compounds are usually needed / to be synthesized in order to successfully develop one new drug?
10,000
Which of the following binding forces is/are involved in drug-target interactions?
I. Hydrophobic interactions
II. Hydrogen bonding interaction
III. Covalent bond
I, II, and III
How many subjects are typically involved in a phase I clinical trial?
10-20
What determine(s) the physico-chemical properties of a drug molecule?
I. Kind of atoms present in it.
II. Number of atoms present in it.
III. Arrangement of atoms present in it.
I, II, and III
How much money does it take to put one new drug in the market?
$1,000,000,000
Thalidomide is still currently used in the clinic despite the fact that it was withdrawn as a sedative in 1960s.
true
Select All That Apply. Which of the following physico-chemical properties is/are related to how a drug molecule behaves in a biological system?
a. stereochemistry
b. hydrogen bonding potential
e. partition coefficient
Select All That Apply. Which of the following types of drugs can be absorbed in the stomach?
d. Drugs containing a carboxylic acid
e. Drugs containing neither acidic or basic functional groups
For atomic orbitals at the same energy level, electrons will populate each orbital until all have one electron, then begin pairing up.
This is known as what?
Hund's rule
Pepto-Bismol has
I. a pink color from food coloring added for branding purposes.
II. Bismuth Subsalicylate as its active ingredient.
Ill. anti-inflammatory activity.
I, II, and III
Ozone
I. Is more stable than dioxygen (O2).
II. Is more effective than dioxygen (O2) in filtering out the damaging UV rays of the sun from reaching the earth.
III. Does not leave any harmful residue in water after treatment of municipal water.
II and III only
Which of the following can be used as an antiseptic?
I. Hydrogen peroxide
II. lodine
III. Potassium carbonate
I and Il only
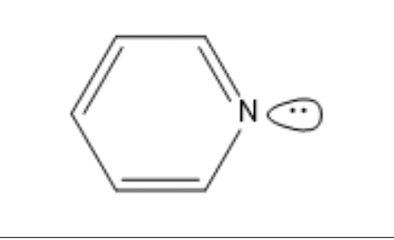
The lone pair on the nitrogen in pyridine does not contribute to the aromaticity of the molecule.
This is because:
The lone pair is perpendicular to the ring m-system.
Inorganic antacids
I. are all weak acids.
II. offer slow but long-term relief of heartburn
Ill. can be combined with an H2 blocker to offer fast and long-lasting acid
reducing properties.
III only
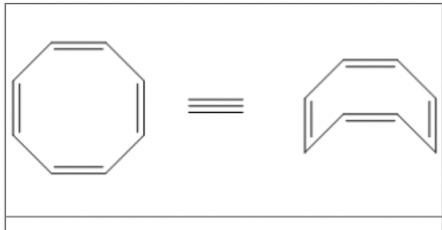
Cyclooctatetraene is an example of a what?
Non-aromatic molecule
Select All That Apply. lodine added to iodized salt can help prevent
c. mental retardation
e. goiter
Which statements about bonds are true?
I. They determine the shape of a drug molecule.
II. They determine the reactivity of a drug molecule.
Ill. They can affect the portioning of a drug molecule between two phases.
IV. They can affect acid-base properties of a drug molecule.
all of the above
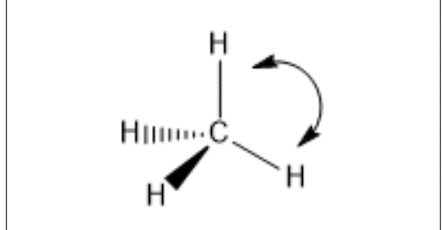
Methane is an example of a molecule that is sp hybridized.
The bond angle will be what?
109.5 degrees
The cyano group (-CN) is an example of what?
an electron withdrawing group
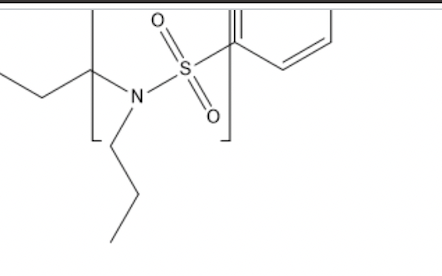
Probenicid (shown above) is used for the treatment of gout.
The section shown in the brackets is which functional group?
a sulfonamide

Trifluoroacetic acid on the right has a much lower pKa than acetic acid on the left.
This is an example of what kind of effect?
an inductive effect
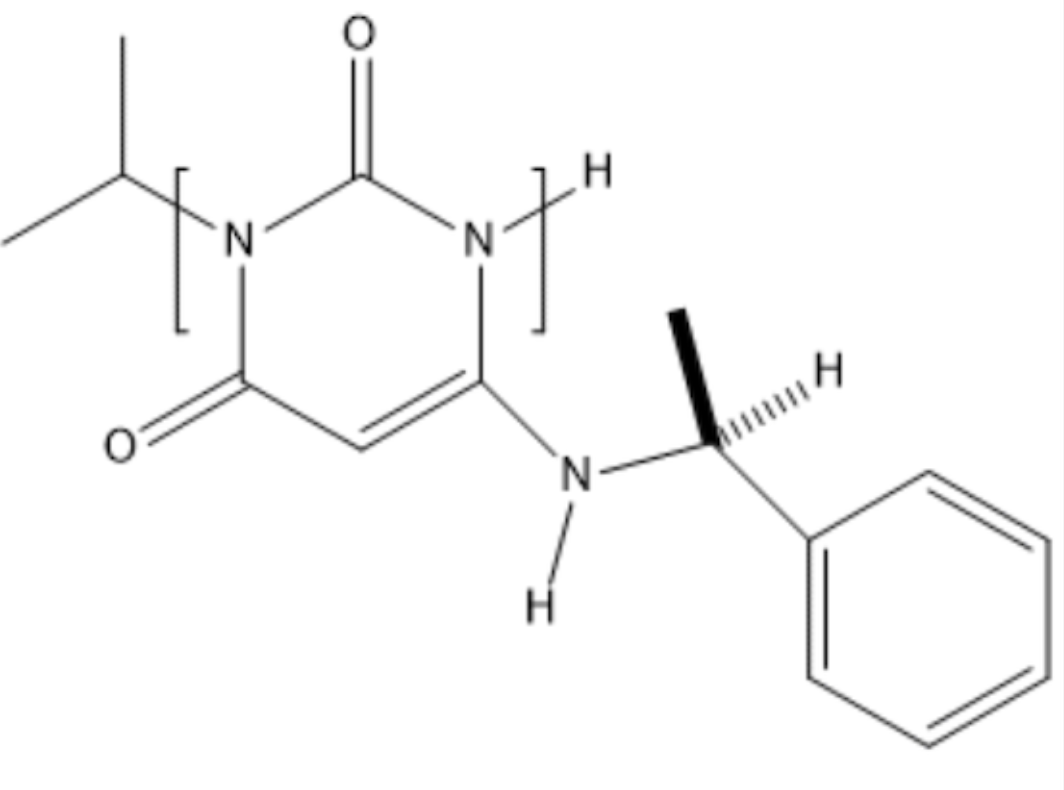
Mavamectin is a drug candidate in phase Ill clinical trials for the treatment of cardiomyopathy.
Identify the functional group shown in the brackets.
a urea
True or False: An aromatic ring such as the phenyl group can act as both an electron donor and as an electron withdrawing group depending on conditions.
true
True or False: A molecule capable of giving up a hydrogen atom is said to be a Bronsted-Lowry base.
false
A molecule that can act as both an acid and a base is said to be what?
amphoteric
According to the Lewis Acid-Base concept, a molecule that can donate a pair of electrons is said to be what?
a lewis base
The pKa is a measure of acid strength. A low pKa indicates a molecule is what?
a strong acid
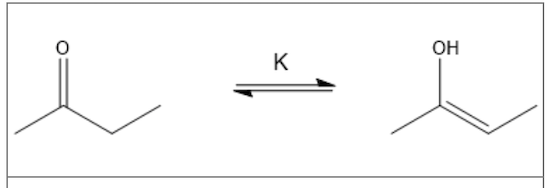
An equilibrium can exist between the ketone and enol forms of 2-butanone.
This effect is known as what?
tautomerization
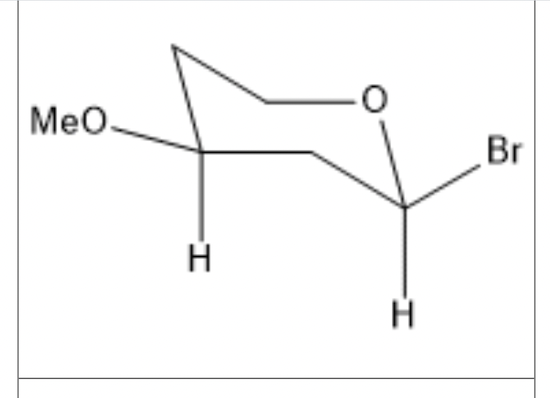
For the cyclohexane ring shown above, are the two groups cis or trans?
cis
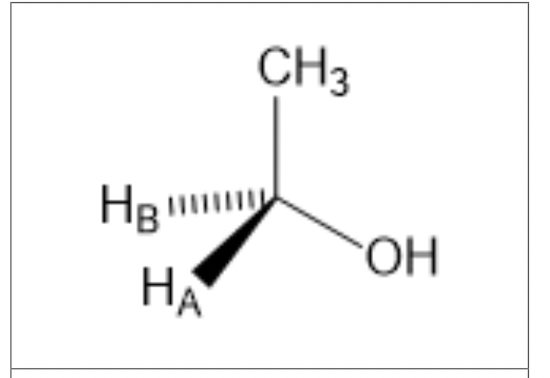
The molecule above has two hydrogen atoms that are not equivalent in a chiral environment. This makes the molecule what?
prochiral
True or False:
A negative πx Value suggests a substituent makes a compound more water soluble.
true
A drug that has a large positive logP value is what?
more soluble in the n-octanol (lipid)/organic layer
The preferred conformation of cyclohexane is what?
the chair
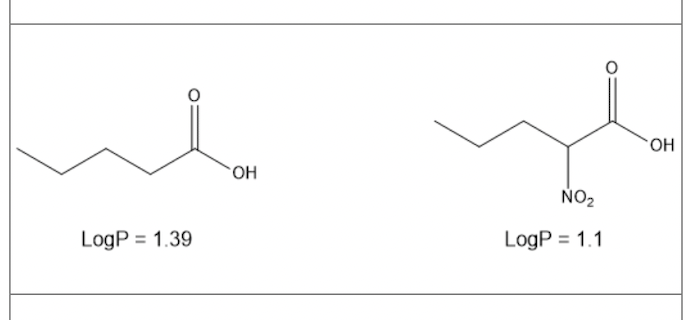
Below are valeric acid and 2-nitrovaleric acid. LogP values are given for both. calculate the pi-value for the nitro group nitrovaleric acid
-0.29
True or False:
Under the Cahn-Ingold-Prelog rules, Bromine would be given a higher priority over oxygen due to its higher atomic number.
true
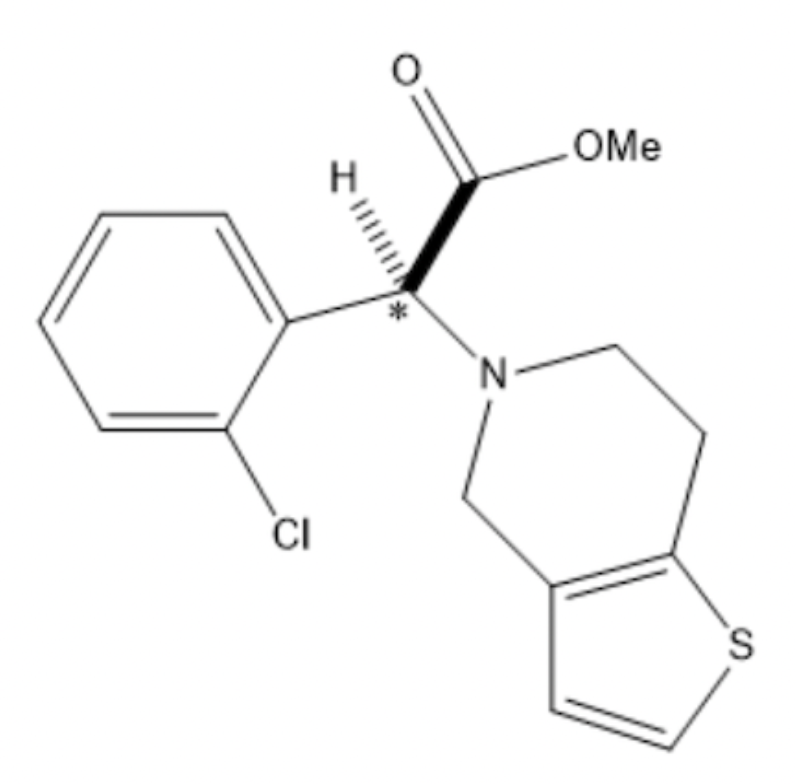
For the drug Clopidogrel, assign stereochemistry to the carbon atom that is marked with an asterisk.
S
Isomers that are non-superimposable, non-mirror images are called what?
diastereomers
True or False:
The Hansch partition coefficient (πx) is a quantitative measure of the effect of a substituent on the partition coefficient of a molecule as a whole.
true
Select All That Apply. Which of the following enzymes is/ar involved in the metabolism of norepinephrine?
a. aldehyde dehydrogenase
b. catechol O-methyl transferase
d. monoamine oxidase
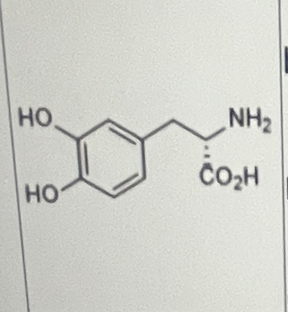
Which of the statements is/are true about the following molecule?
I. It can cross the blood-brain barrier
II. It is a substrate of a peripheral decarboxylase
III. It is a substrate of a decarboxylase in the CNS
I, II, and III