Molar mass (Ch 7.3)
0.0(0)
Get a hint
Hint
Formula 1: n = m / M
Get a hint
Hint
n = no. mol, m = sample mass (g), M = g/mol^(-1)
Get a hint
Hint
Formula 2: n = N / Na
Get a hint
Hint
n = no. mol, N = no. of particles, Na = avocado
Card Sorting
1/3
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
1
New cards
Formula 1: n = m / M
n = no. mol, m = sample mass (g), M = g/mol^(-1)
2
New cards
Formula 2: n = N / Na
n = no. mol, N = no. of particles, Na = avocado
3
New cards
Percentage Composition
% of element in :
[(amount of atom x atom mass) / molar mass (g.mol^-1)] x 100
4
New cards
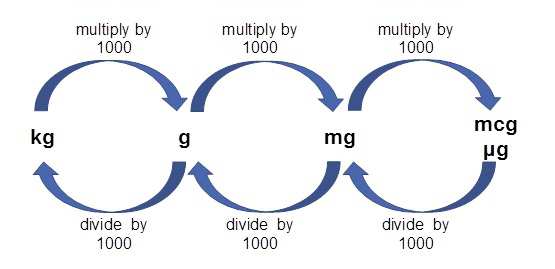
Unit conversions