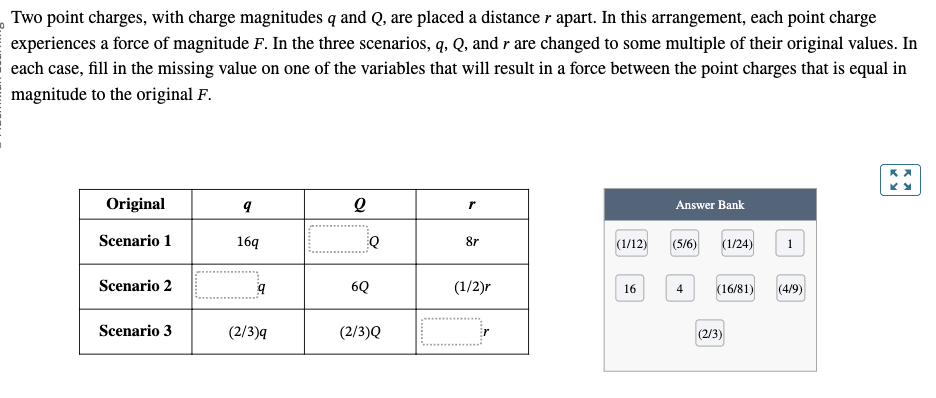
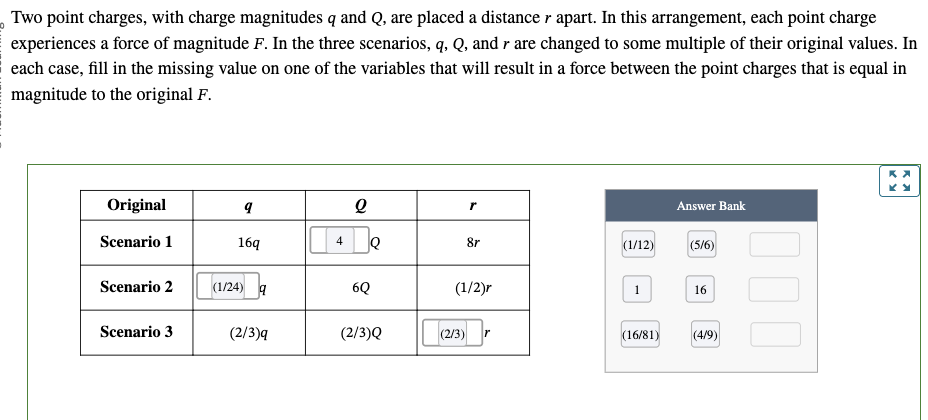
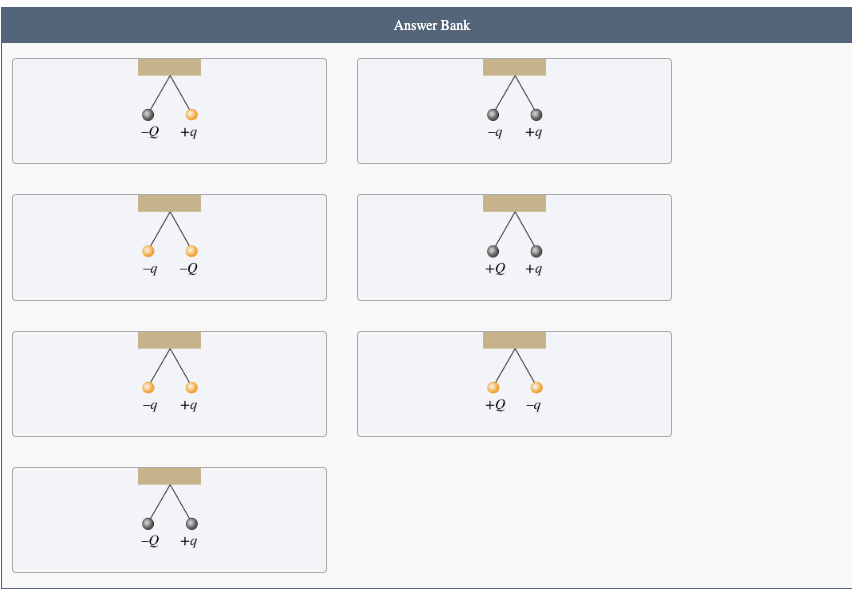
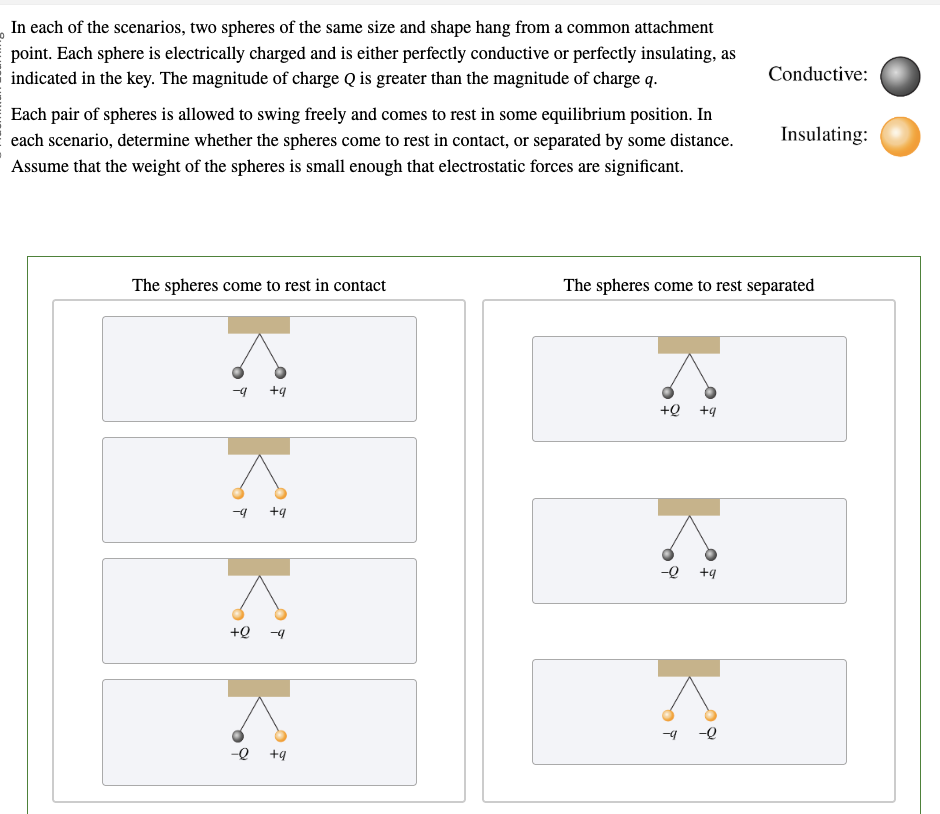
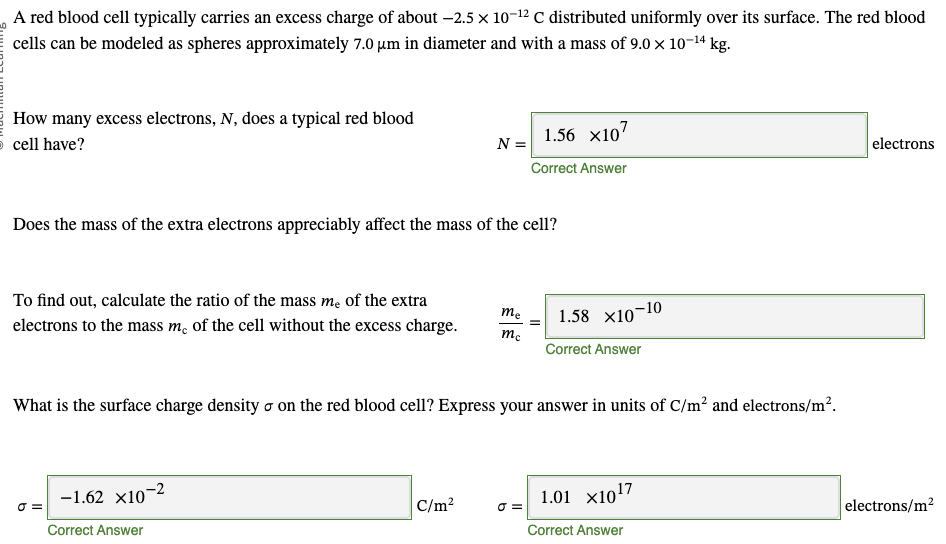
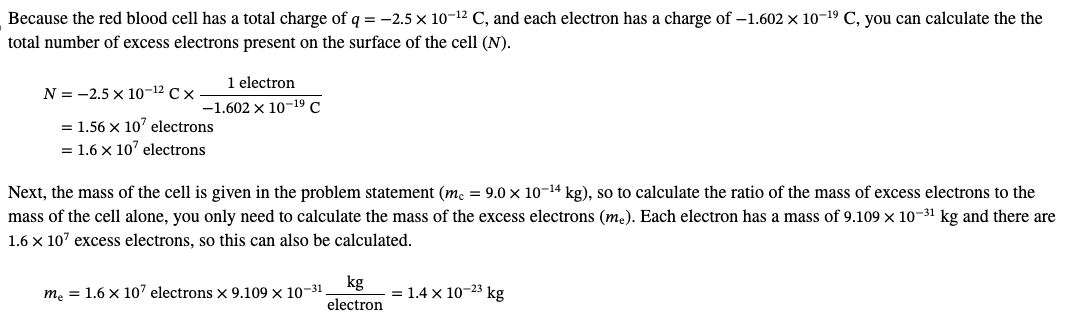
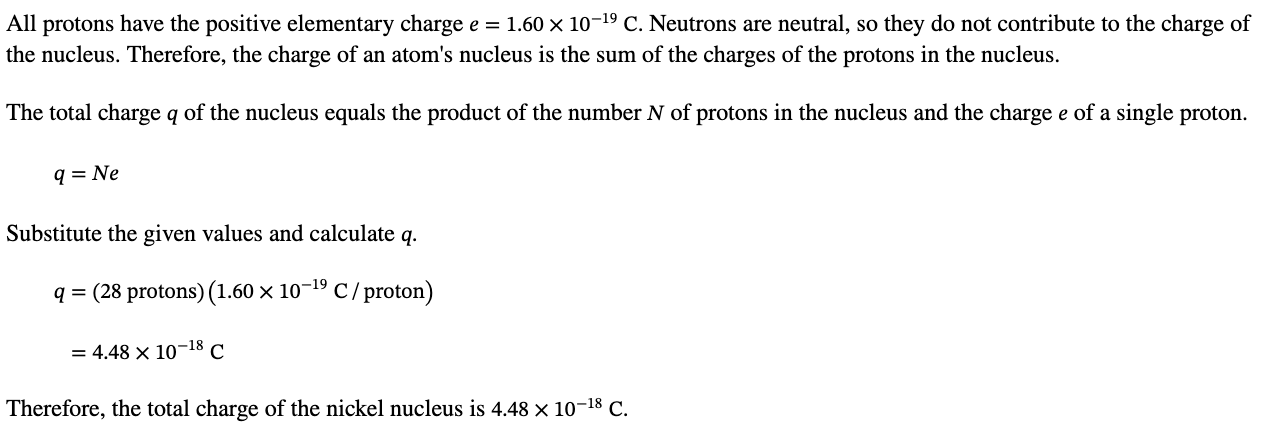
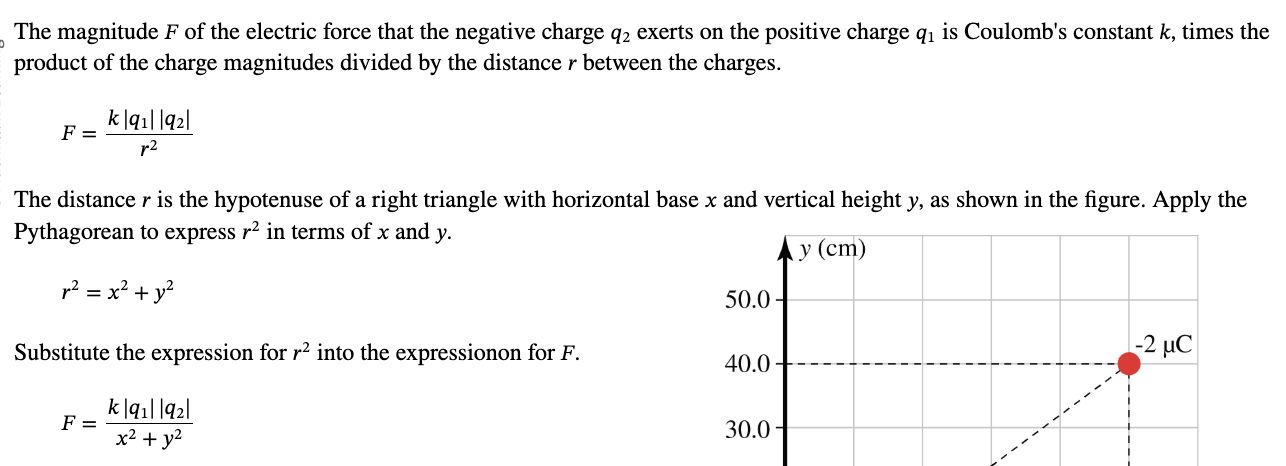
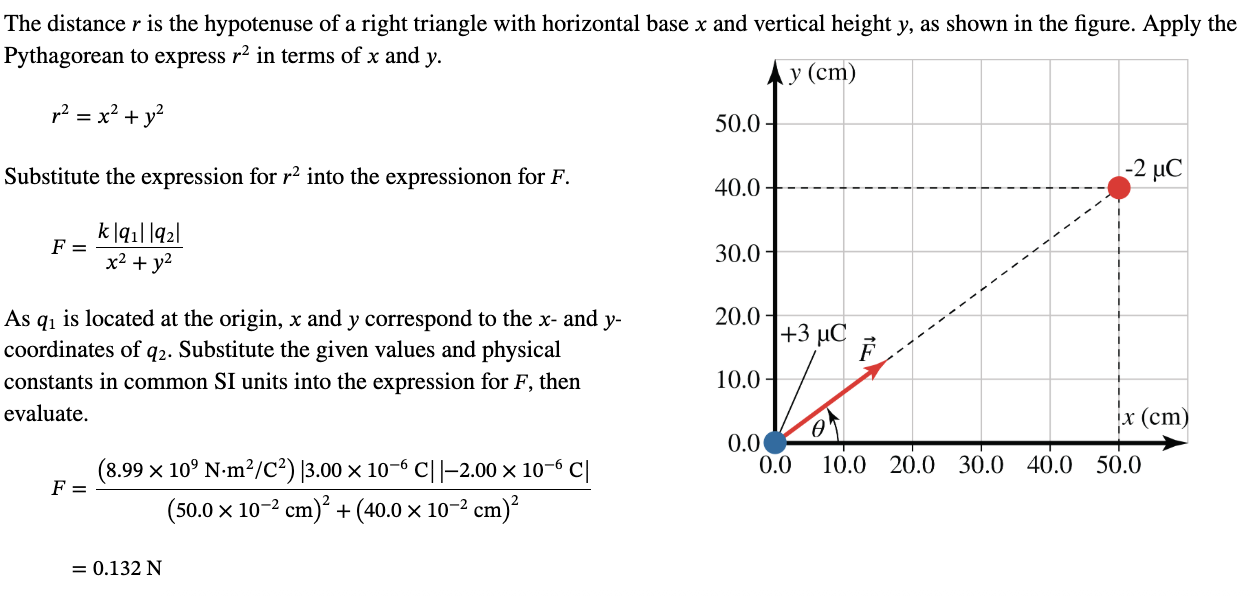
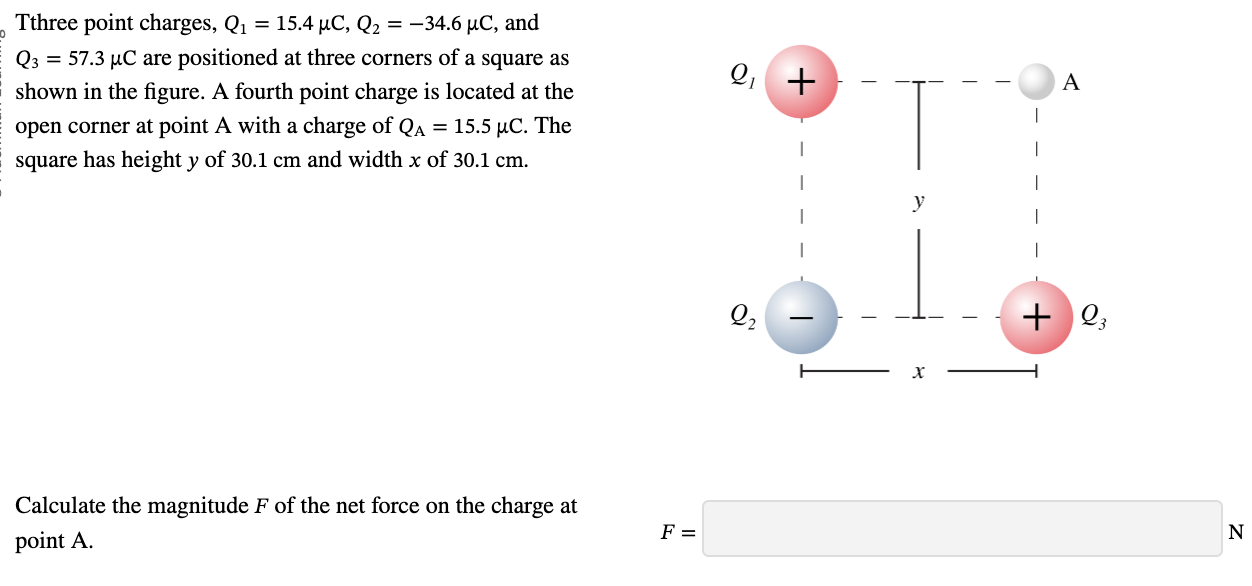
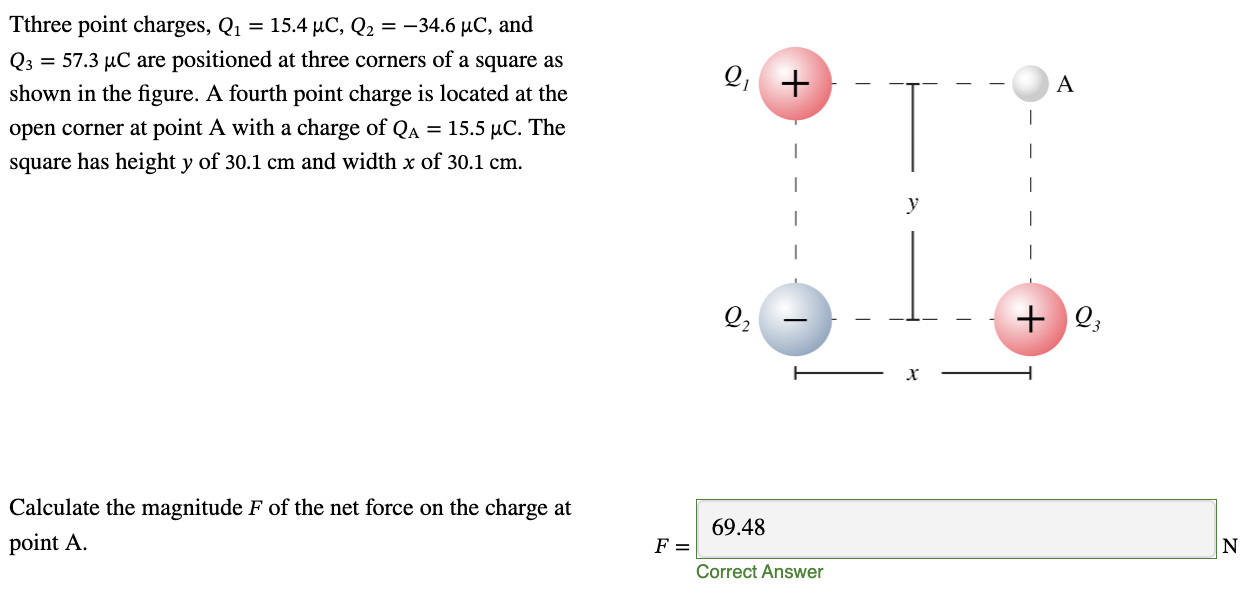
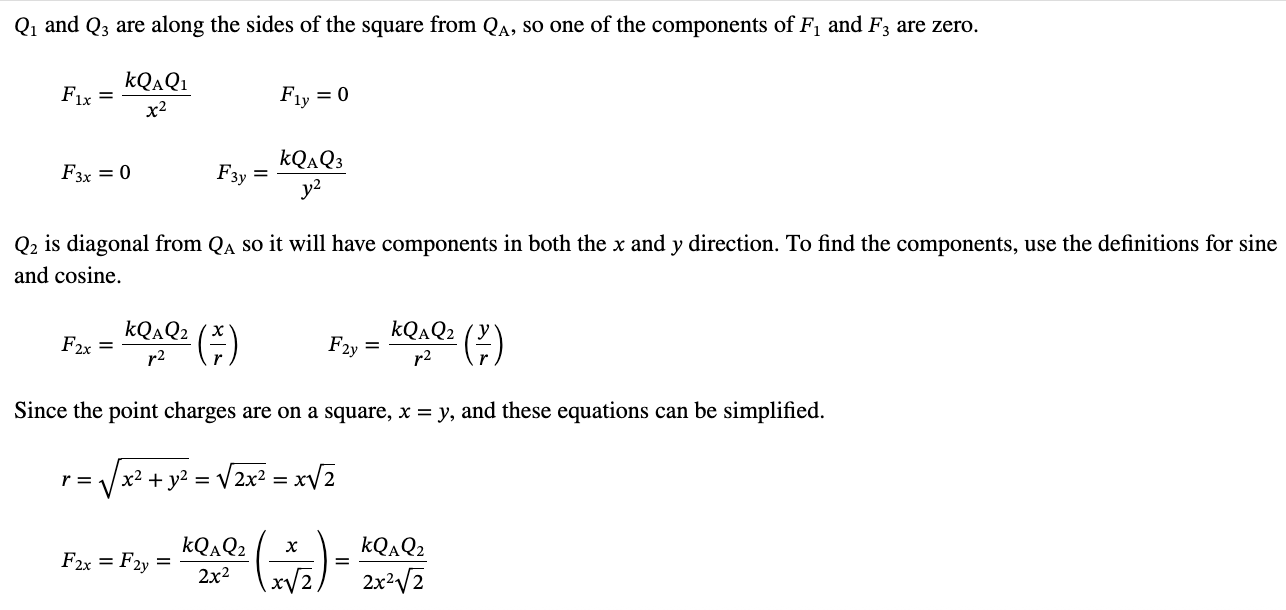
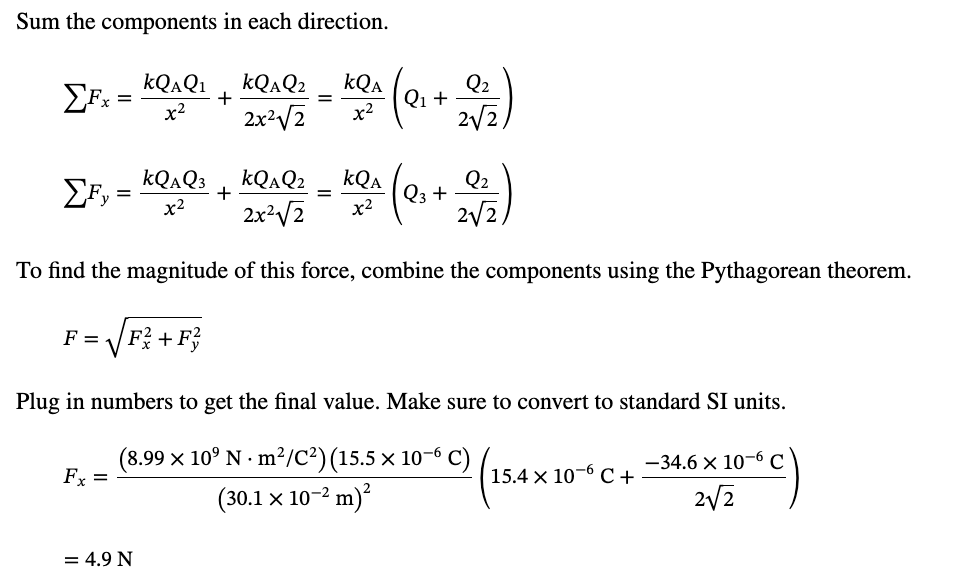
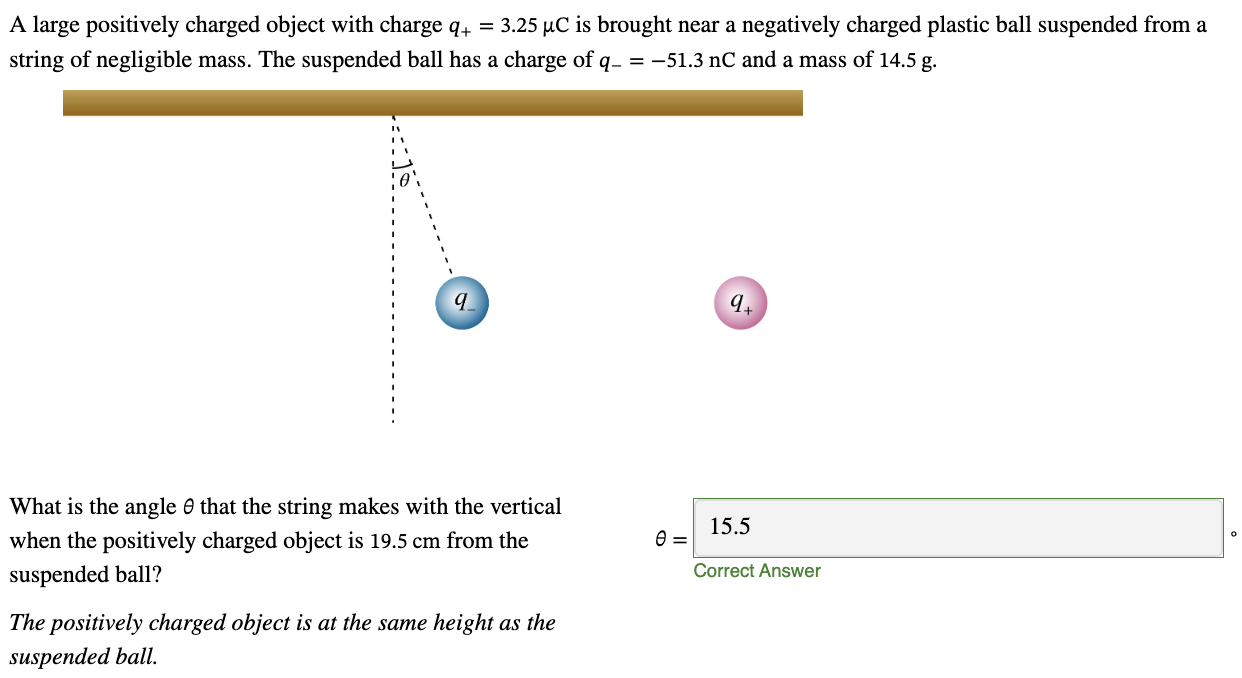
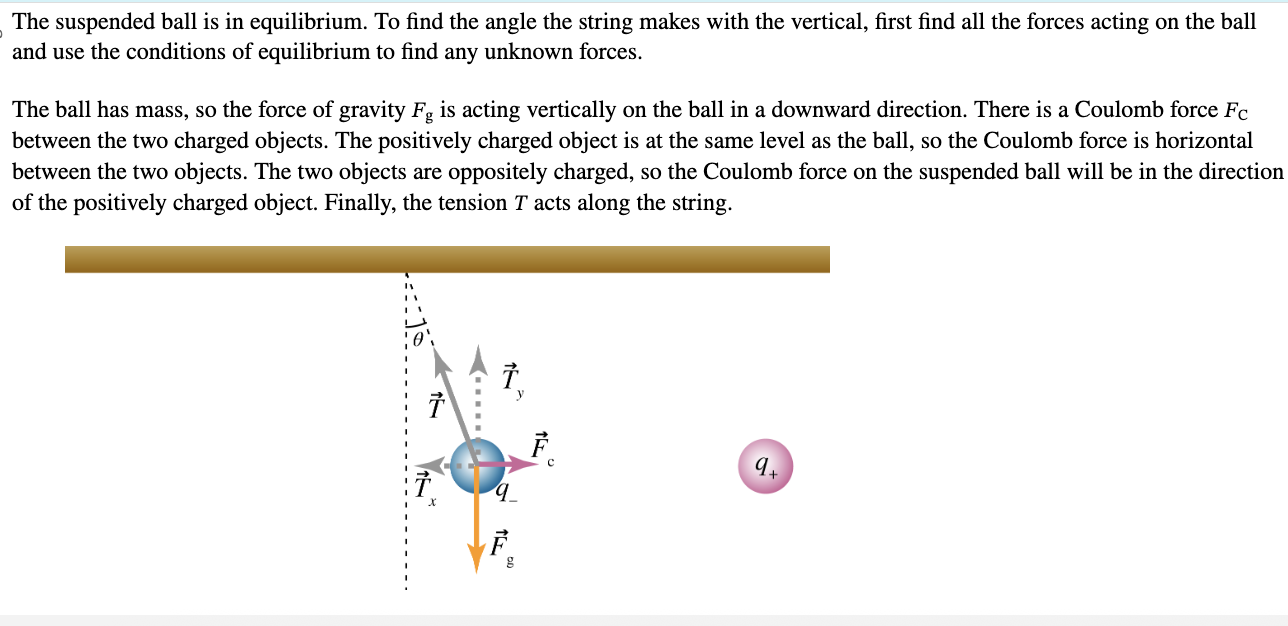
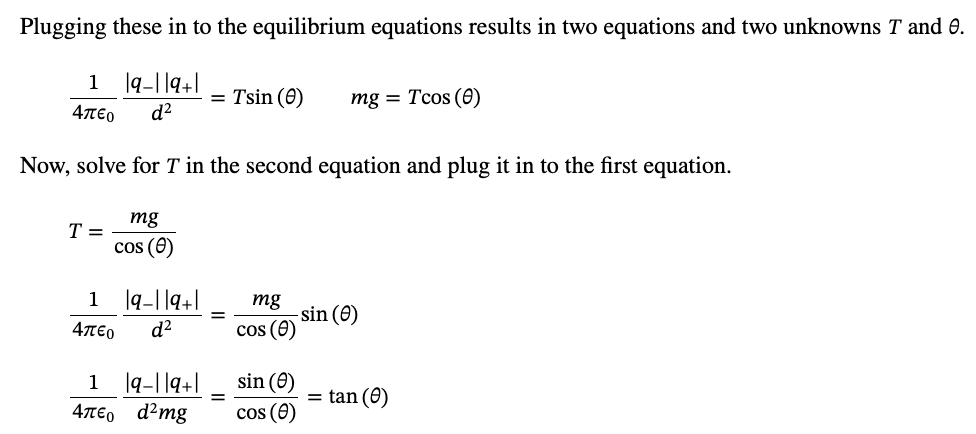
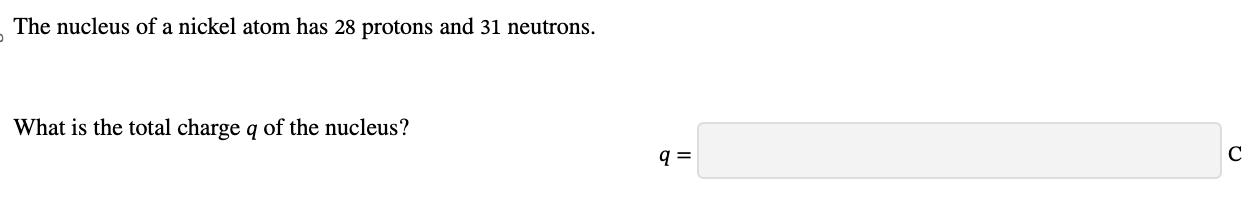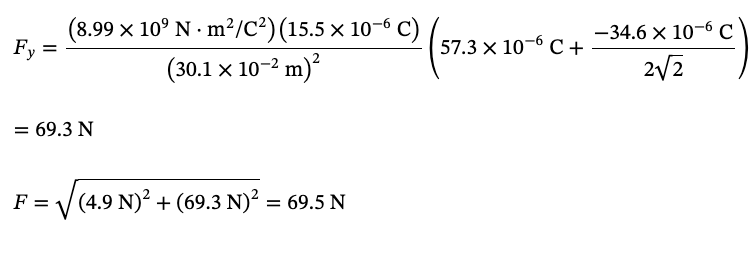PHYS 0040 ONLINE+WRITTEN HW (9+4)=13
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
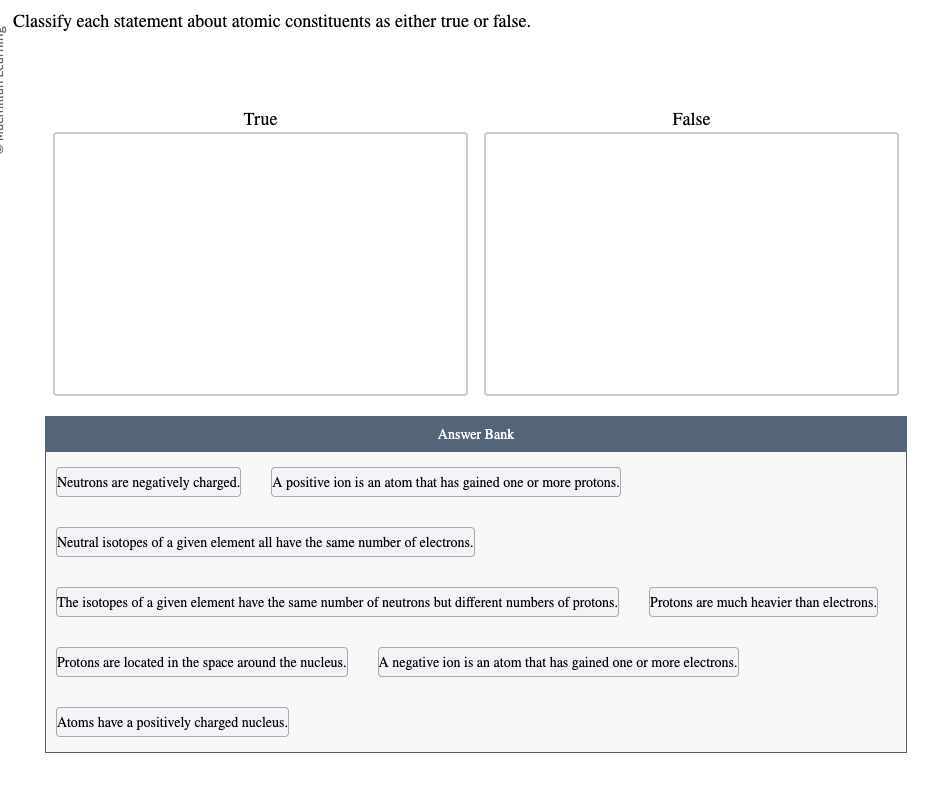
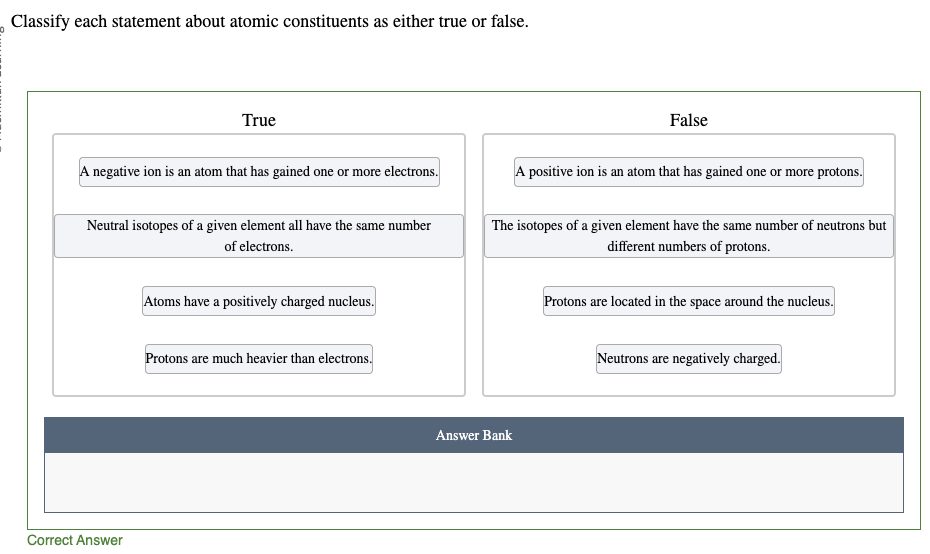
A negative ion has gained one or more electrons. A positive ion has lost one or more electrons. An atom will not gain or lose protons to become an ion, because the protons are located in the nucleus.
Atoms have a positively charged nucleus with electrons orbiting around them. The positive charge of the nucleus comes from the positively charged protons. Neutrons are also in the nucleus, but they have no charge.
Isotopes of an element all have the same number of protons. Because the number of electrons has to match the number of protons in order for the atoms to be neutral, they all also have the same number of electrons as long as the isotopes are neutral. Isotopes differ because they have different numbers of neutrons.
Protons are much heavier than electrons. Each proton weighs about the same as 1800 electrons.

x


x

x
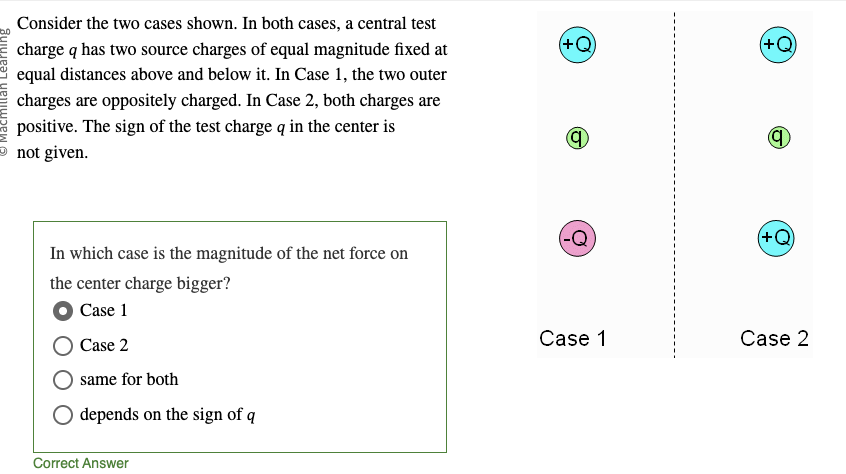

x
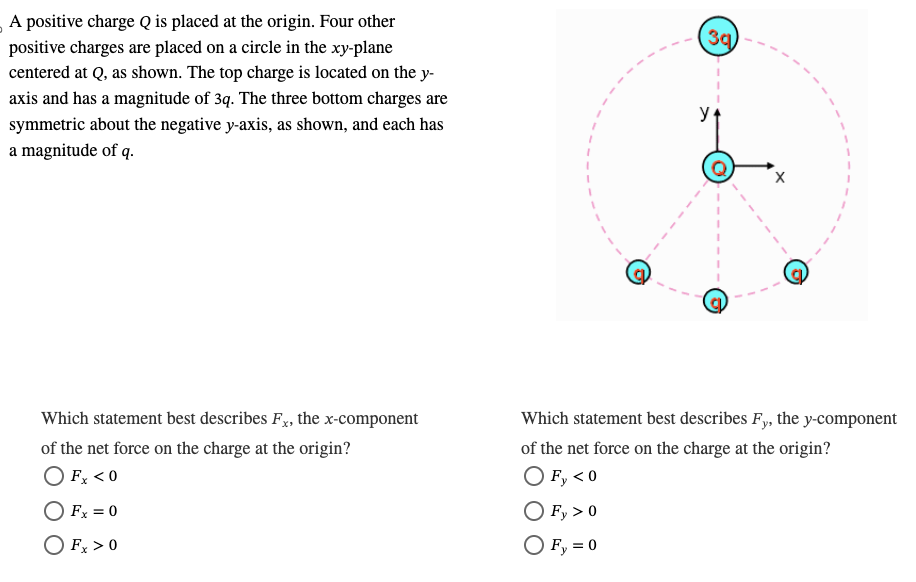
x
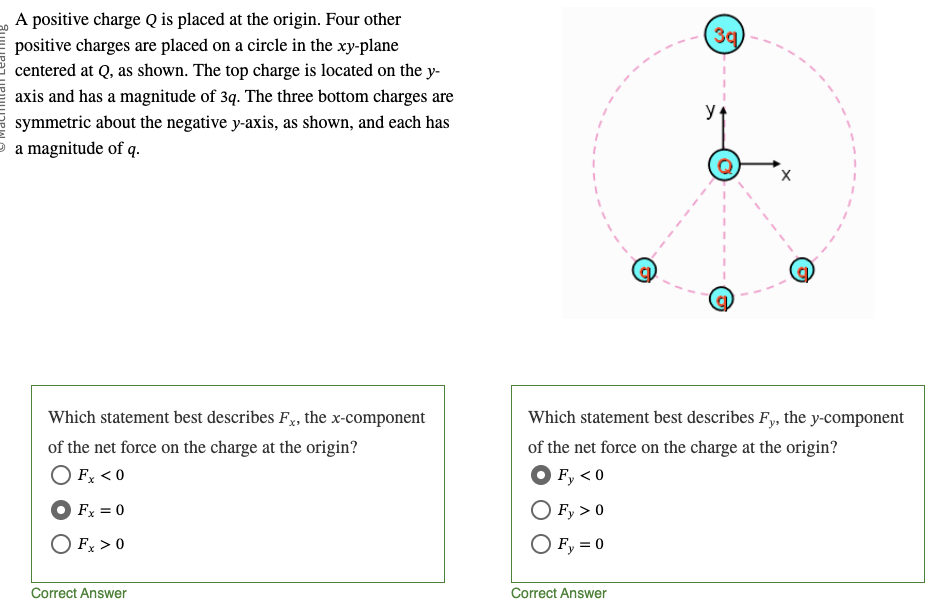


x
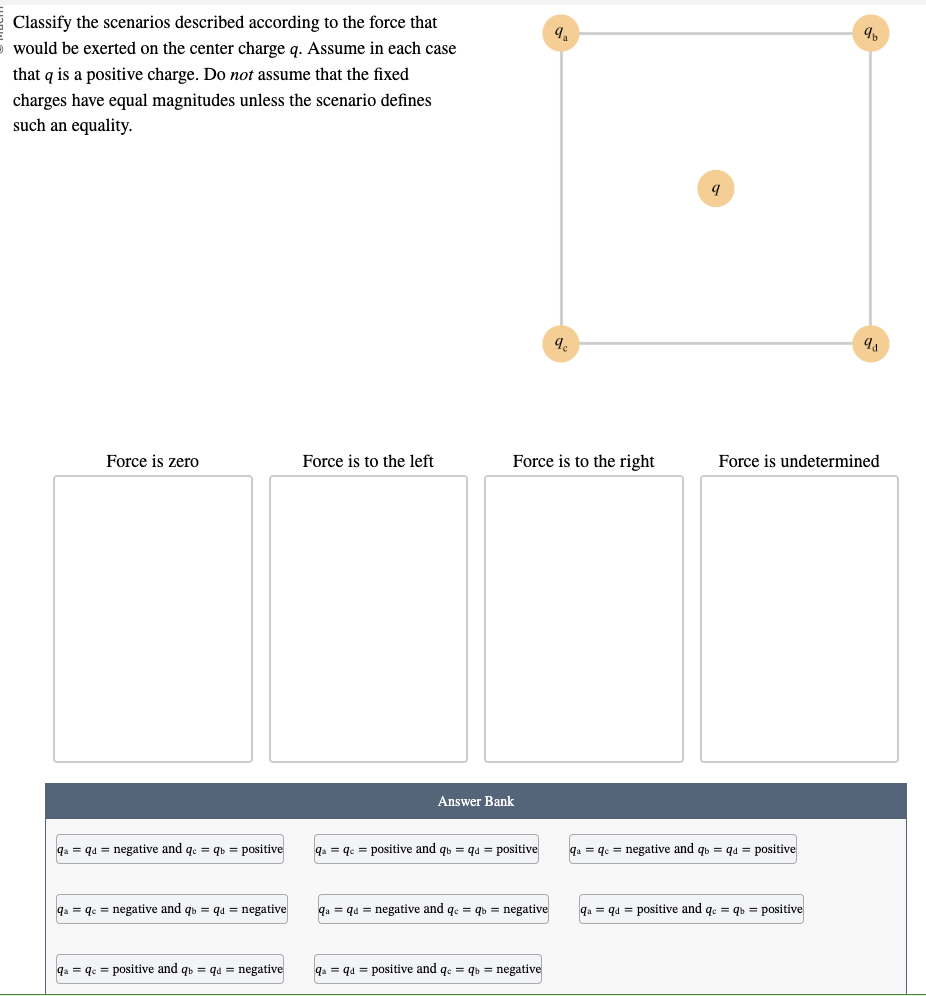
x

x