Monosaccharide reactions, oligosaccharides and polysaccharides
0.0(0)
Card Sorting
1/52
Earn XP
Description and Tags
Last updated 1:29 PM on 5/3/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
53 Terms
1
New cards
hydroxy functional group reactions
can be converted into esters or ethers or can be oxidised to aldehydes, ketones or carboxylic acids
2
New cards
carbonyl functional group reactions
can react with nucleophiles or can be reduced or oxidised
3
New cards
reactivity of C1 (anomeric carbon) in cyclic sugars
Anomeric carbon is the most reactive, as it has two C-O single bonds. It’s prone to nucleophilic attack because of the oxygens’ electron withdrawing effect.
4
New cards
glucose acetal formation
hemiacetal monosaccharides react with alcohols and acid to give acetals at the anomeric centre
5
New cards
naming monosaccharide acetals
glycosides. Exact name determined by the monosaccharide they’re derived from eg glucose acetals are *glucosides*
6
New cards
glycoside hydrolysis
Presence of 2 O at anomeric centre means glycosides undergo hydrolysis in presence of acid to give a hemiacetal sugar and alcohol (aglycone).
7
New cards
What are reducing sugars?
sugars with an OH group at the anomeric centre (hemiacetals). The hemiacetal group is in equilibrium with the open chain form (aldehyde or ketone).
8
New cards
Tollens reagent
Solution of ammoniacal silver nitrate (contains Ag(I)). Adding a reducing sugar forms silver metal which forms a silver mirror
9
New cards
Benedict’s reagent
Aqueous solution containing Cu(II). On reaction with a reducing agent, copper (I) oxide (Cu2O) is formed as a brick-red precipitate
10
New cards
How was Benedict’s reagent used in medicine?
can be used to detect elevated glucose levels in urine to diagnose diabetes
11
New cards
Non reducing sugars and oxidising agents
Don’t react. They are acetals at the anomeric centre (glycosides)
12
New cards
oxidation of sugars
Oxidation of primary alcohol in an aldose gives -uronic acid eg oxidation of D-glucose at C6 gives D-glucuronic acid. These undergo intramolecular esterification to give lactones eg ascorbic acid.
13
New cards
oligosaccharides
polysaccharides that hydrolyse to give 2 - 10 monosaccharides. Often associated with proteins and lipids.
14
New cards
Most common oligosaccharides
Disaccharides - the two component monosaccharides can be the same or different. Disaccharides contain an O-glycosidic (acetal) bond between C-1 on one sugar and any hydroxy group on the second sugar.
15
New cards
starch hydrolysis
Diastase (enzyme) hydrolysis of starch gives the disaccharide maltose.
16
New cards
cellulose hydrolysis
Partial chemical hydrolysis of cellulose gives the isomeric cellobiose.
17
New cards
structure of maltose and cellobiose
composed of two glucose units linked by a 1-4’ glycosidic linkage. The anomeric carbon C-1 is an alpha-anomer (maltose) or beta-anomer (cellobiose).
18
New cards
lactose
Disaccharide which only naturally occurs in milk, concentration species dependent from 0 to 7%. Made up of D glucose and D galactose
19
New cards
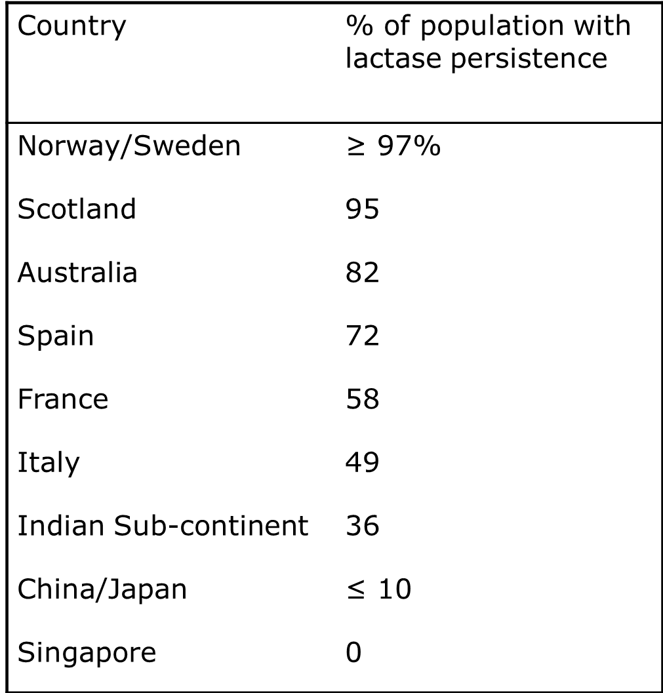
lactose intolerance
Many adults have low levels of intestinal enzyme beta-D-galactosidase enzyme. Ingested lactose moves to the colon, where bacterial fermentation produces CO2, H2 and organic acids.
20
New cards
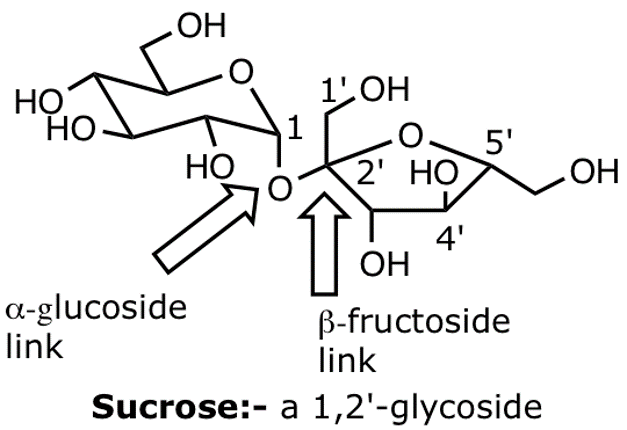
sucrose
Most naturally abundant disaccharide, used as table sugar. Not a reducing sugar and doesn’t undergo mutarotation as there’s no hemiacetals
21
New cards
sucrose hydrolysis
gives a mixture of D-glucose and D-fructose called ‘invert sugar’. Sucrose \[a\]D = + 66.6. Invert sugar \[a\]D = - 22. Enzyme catalysing the process =beta-D-fructofuranoside (‘invertase’)
22
New cards
producing invert sugar in cooking
heating sucrose with a little lemon juice/cream of tartar
23
New cards
what are polysaccharides?
*glycans* are carbohydrates with 10’s, 100’s or 1000’s of simple sugars linked by glycosidic bonds.
24
New cards
heteropolysaccharides
composed of more than one type of monosaccharide unit
25
New cards
homopolysaccharides
Most common. Composed of just one type of monosaccharide unit.
26
New cards
glucans
glucose homopolysaccharides
27
New cards
galactans
galactose homopolysaccharides
28
New cards
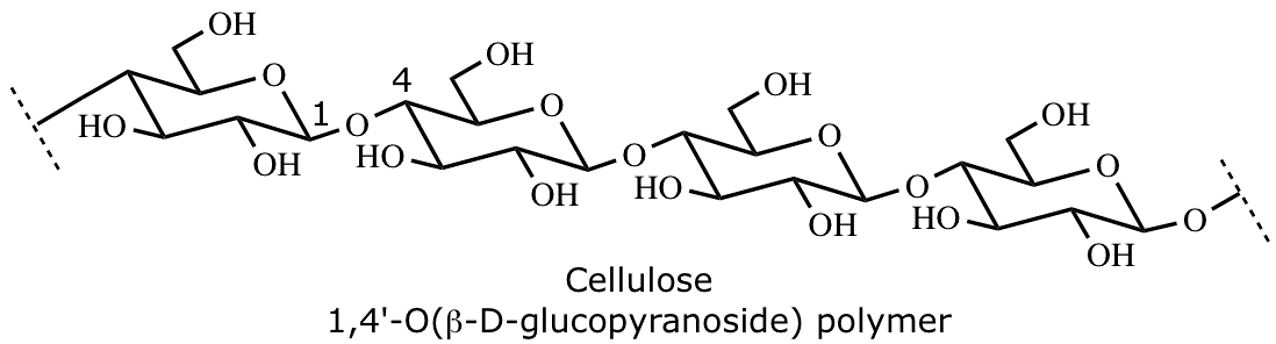
cellulose
Linear structural polysaccharide, the most abundant organic molecule in the biosphere. Composed of D glucose units joined by 1, 4 beta glycosidic linkages
29
New cards
cellulase
Higher animals eg humans don’t have the enzymes that hydrolyse 1,4’-β-linkages, so cellulose can’t be used ‘directly’ as a food source.
30
New cards
starch
Starch and its derivatives are the second most abundant polysaccharide, found in plants and animals. Found in microscopic plant granules
31
New cards
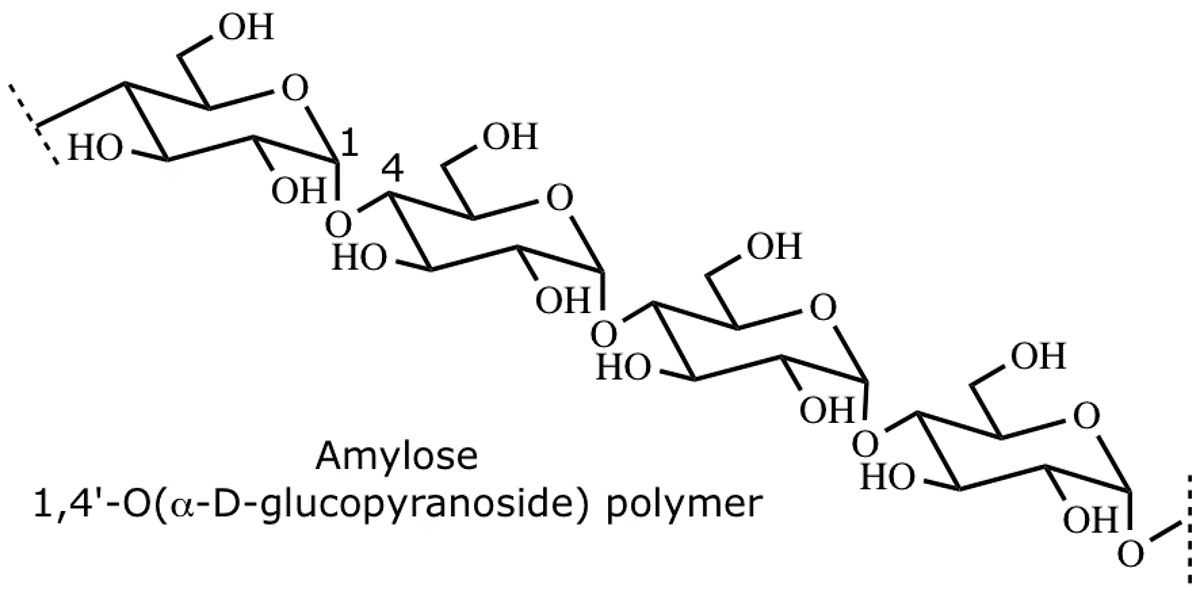
amylose
has between 10^2 and 10^3 D-glucose monomers linked by 1,4’-a-glycosidic bonds.
32
New cards
digestion of starch
It’s hydrolysed by glycosidase enzymes which hydrolyse alpha-glycosidic links.
33
New cards
Why is D glucose stored as a polymer rather than as a large number of monomer units?
To avoid large osmotic pressures. Osmotic pressure from 1000 aqueous glucose monomers would be 1000 times 1 amylose molecule with 1000 glucose units linked together which would lead to cell membranes rupturing.
34
New cards
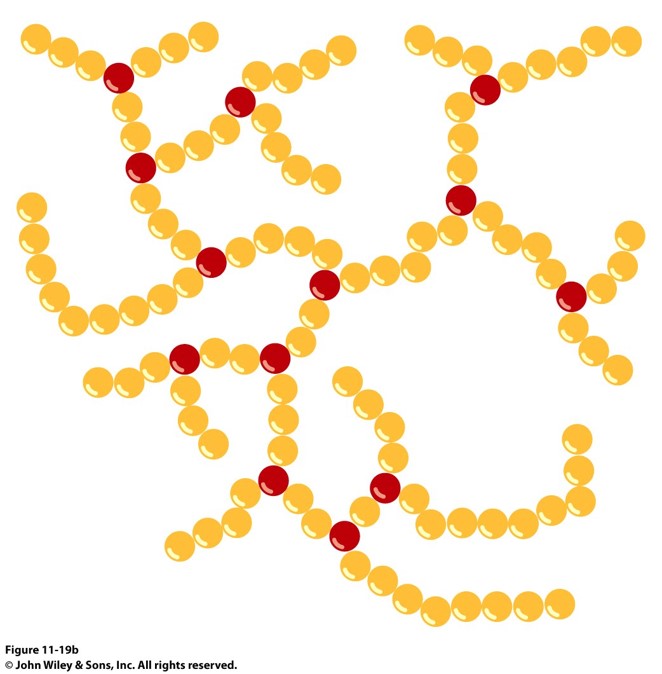
amylopectin
branched polysaccharide. Contains 1,4’-alpha and 1,6’-alpha-glycosidic linkages (responsible for the branching). Distance between branch points is 24 to 30 glucose units.
35
New cards
glycogen
Energy storage glucose polysaccharide in animals containing 1,4 and 1,6 alpha glycosidic links. Highly branched with 1,6 glycosidic links every 6 to 8 glucose units.
36
New cards
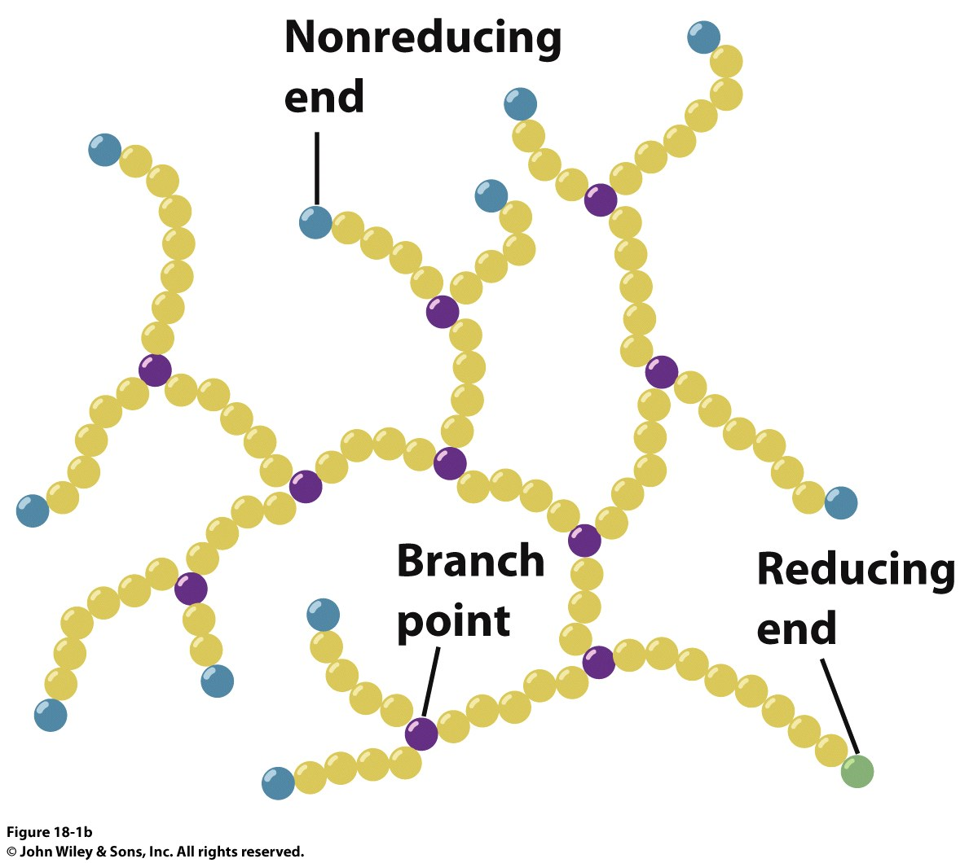
how is glycogen degraded for metabolic use?
Glucose phosphorylase hydrolyses the terminating group and adds a phosphate → glucose-1-phosphate. Glycogen phosphorylase can’t cleave linkages closer than four glucoses from a branch point.
37
New cards
glycogen metabolism
Human metabolism consumes 160g carbs/day. 75% is consumed as glucose by the brain, and is the brain’s only fuel except upon prolonged starvation. Most of carbohydrate intake is via starch
38
New cards
glycogen debranching enzyme
Transfers 1,4’-alpha-linked trisaccharide unit from a ‘limit branch’ to the non-reducing end of another branch. Remaining 1,6’-alpha-linked glucose hydrolysed by the same enzyme
39
New cards
polysaccharide secondary structure
Results from local conformational variety due to rotations about the single bonds involved in glycosidic linkages. Glycosidic bond nature important as certain angle combinations are more stable
40
New cards
angles of 1,4 glycosides in alpha links
have 2 torsional angles to consider, phi and theta. In alpha links this results in a gentle turn that produces a helix when extended.
41
New cards
benefit of sugar units rotating 180 to the next in beta chains
reduces steric repulsion and maximises number of H bonds able to form
42
New cards
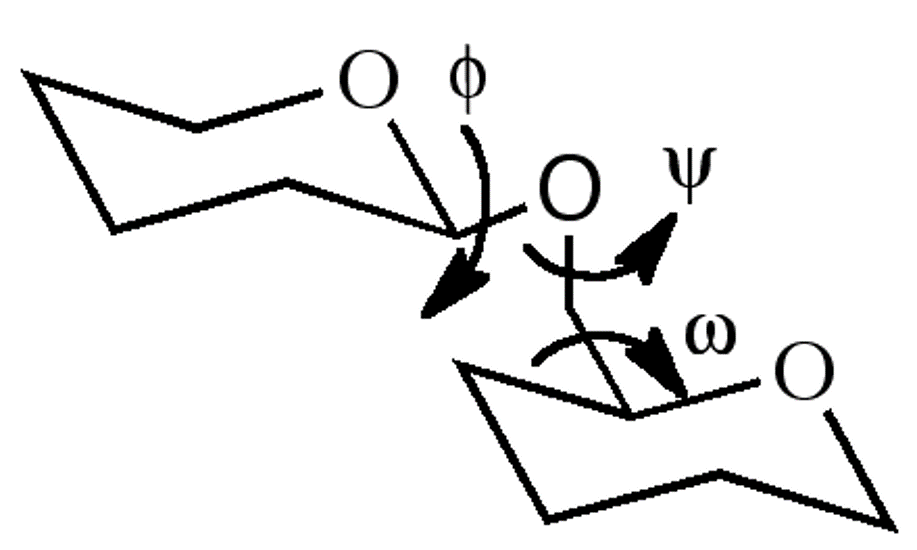
1,6’ glycoside torsional angles
three angles to consider, phi, theta and w : there’s a much wider range of conformations available
43
New cards
polysaccharide tertiary structure
concerns the way the entire polysaccharide backbone is arranged in three dimensional space.
44
New cards
polysaccharide quaternary structure
concerns the way polysaccharide chains aggregate with other polysaccharide chains eg ribbons forming sheets and sheets stacking on top of each other.
45
New cards
What dictates how the chain folds and packs together?
non-covalent , long-range interactions between the functional groups present in the monosaccharide units eg hydrogen bonding or charge-charge interactions
46
New cards
ribbons
linear arrangement of beta linked glucose units in cellulose/N-acetylglucosamine units in chitin. Uniform distribution of hydroxy groups on outside of chains.
47
New cards
cellulose ribbons and hydrogen bonding
Ribbbon stabilised by intramolecular H bonds between OH groups on adjacent glucoses. They associate laterally to form sheets stabilised by intermolecular H bonds. Inter-sheet hydrogen bonding → further association.
48
New cards
flexible helices
Alpha-glycosidic bond results in chains with wide hollow helix stabilised by H bonding
49
New cards
How does the iodine-starch test work?
Small molecules can be accommodated into polysaccharide helices’ central cavities to give inclusion complexes. Aqueous solution of I2 and I- forms a blue-violet colour starch inclusion complex.
50
New cards
why is the blue-black colour produced in the starch-iodine test?
arises from charge-transfer interactions between the rows of triiodide anions, \[I3\]-, arranged end-to-end in the amylose cavity.
51
New cards
composition of starch
amylose (insoluble in cold water, 20 % of starch) and amylopectin (soluble in cold water, 80 % of starch)
52
New cards
Angles of 1,4 glycosides in beta links
2 torsional angles to consider, phi and theta. For beta links it results in a zig zag pattern with sugars rotated by 180 degrees in relation to the next. forming ribbons
53
New cards
advantage of beta ribbons
When two or more cellulose/chitin chains make contact, OH groups are ideally arranged to make hydrogen bonds.