electronic configuration
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
number of orbitals in each sub shell
s - 1
P - 3
d - 57
f -
what are orbitals
an orbital is the space which an electron moves in
orbitals in the same sub-shell have the same energy
there are two electrons in each orbital which spin in opposite directions
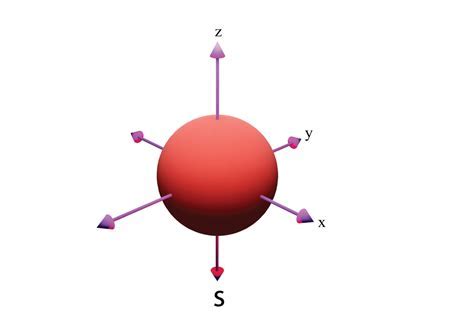
Shape of S orbital
spherical
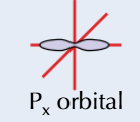
shape of P orbital
dumbbell shape
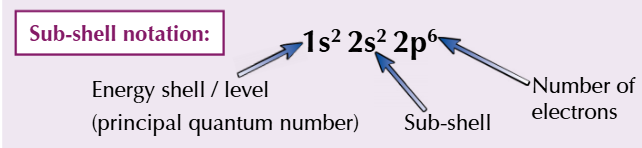
sub-shell notation electronic configuration of neon
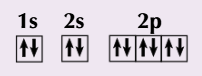
Electronic configuration using electrons in boxes
each box represents one orbital and each arrow shows one electrons
the arrows point in opposite directions as the electrons have opposite spins
order of filling the sub-shells
1s,2s,2p,3s,3p,4s,3d,4p,4d,4f
electronic configuration of chromium
(Ar) 4s1 3d5
electronic configuration of copper
(Ar) 4s1 3d10