quiz 1 - hybridization
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
what is the hybridization, shape, and bond angle for 4 sigma bonds
sp3, tetrahedral, 109.5°
what is the hybridization, shape, and bond angle for 3 sigma bonds, 1 lone pair
sp3, trigonal pyramidal, 107°
what is the hybridization, shape, and bond angle for 2 sigma bonds, 1 lone pair
sp2, bent
what is the hybridization, shape, and bond angle for 3 sigma bonds
sp2, trigonal planar, 120°
what is the hybridization, shape, and bond angle for 2 sigma bonds
sp, linear, 180°
what combinations of sigma bonds and lone pairs ALWAYS result in polar molecules?
3 sigma bonds, 1 lone pair
2 sigma bonds, 2 lone pairs
in molecules with 4 sigma bonds, if all of the atoms/groups attached to the central atom identical, is the molecule polar or nonpolar?
nonpolar
if a molecule has 3 sigma bonds and all atoms are identical, is the molecule polar or nonpolar?
nonpolar
if a molecule has 3 sigma bonds and one atom is different while the other two are the same, is the molecule polar or nonpolar?
polar
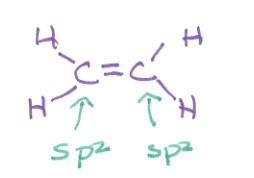
polar or nonpolar and why?
nonpolar due to symmetry
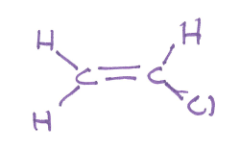
polar or nonpolar and why?
C-Cl is strongly polar and not cancelled by C-H bonds
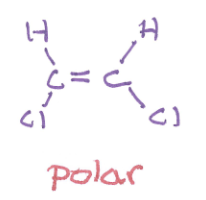
polar or nonpolar and why?
polar, has two C-Cl dipoles point towards the same side, so their dipoles add together instead of cancelling out
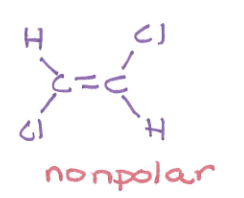
polar or nonpolar and why?
nonpolar, has C-Cl bonds that point in opposite directions, so they cancel
in a lewis structure, which atoms don’t follow the octet rule?
Boron (B) and Aluminum (Al) both have six valence e- (3 sigma bonds)
Beryllium (Be) has four valence e- (2 sigma bonds)
Hydrogen (H) and Helium (He) have 2 valence e- (1 sigma bond)
what is the formula to calculate for formal charge?
formal charge = formal charge = # valence e - # bonds - # nonbonding e
how do you know which bonds in a molecule is shortest?
triple bonds are shortest, single bonds are longest
sp (linear) is shortest, sp3 (tetrahedral) is longest
finally, smaller atom = shorter bond
which atom on the periodic table is the most electronegative?
F
which atom on the periodic table is the largest stable atom?
Cs
which atom on the periodic table is the smallest stable atom?
He
what is the overall formal charge for a neutral molecule?
0
what is the overall formal charge for a charged molecule?
net charge of that ion
ex: overall formal charge of HCOO- is -1