PHYS 1320 Exam 1
1/43
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
44 Terms
What is special about the change in the state function called enthalpy? ΔH
For a process taking place at constant pressure, the change in enthalpy gives the heat added to the system. ΔH = Q
What is important about the change in the state function called internal energy? ΔU
For a process taking place at constant volume, the change in the energy is equal to the heat added to the system. ΔU = Q
What is an isothermal process?
This is a process that takes places at constant temperature. What this means is that the final temperature of the system is equal to the initial temperature of the system.
What is an adiabatic process?
This is a process that takes place in an insulated container so that no heat is exchanged with the surroundings.
What is an isobaric process?
As isobaric process is a process that takes place at constant pressure.
What is an isochoric process?
An isochoric process is a process that takes place at constant volume.
What four steps make up the thermodynamic cycle of the Stirling engine?
Isothermal (constant temperature) expansion at T hot. Isochoric (constant volume) colling to T cold. Isothermal compression at T cold. Isochoric heating back to T hot.
What four steps make up the thermodynamic cycle of the Carnot engine?
Isothermal reversible expansion at T hot, adiabatic reversible expansion to lower the temperature to T cold, isothermal reversible compression at T cold, and adiabatic reversible compression to raise the temperature back to T hot.
What is the difference between a reversible process and an irreversible process?
A reversible process takes place slowly, so that the system is not taken out of equilibrium. An irreversible process takes place quickly, so that the system is taken out of equilibrium. (A truly reversible process would not take place in any finite amount of time.)
The surroundings are always assumed to take on hear (or give off heat) in a reversible fashion. Why?
The surroundings is the “rest of the universe,” an infinite reservoir. Thus when any finite amount of heat from the system is spread throughout the surroundings, the perturbation to the state of the surroundings is negligible. Hence the surroundings remains in equilibrium.
What is the efficiency of a Carnot cycle running between a hot reservoir at T hot and a cold reservoir at T cold?
ε = (T hot - T cold)/ T hot
What postulate concerning energy forms the basis of the first law of thermodynamics?
The energy of the universe remains constant.
True or False? All reversible heat engines operating between two heat reservoirs have the same efficiency.
True, they all have the same efficiency, regardless of the working fluid. The efficiency is the Carnot efficiency.
The efficiency of a Carnot engine operating between two heat reservoirs and using an ideal gas as the working fluid is given by ε = (T hot - T cold)/ T hot. What is the efficiency if the working fluid is some other non-ideal gas?
The efficiency is the same, regardless of the working fluid.
True or False? If a piston expands irreversibly, it is impossible to calculate the work because it is impossible to determine the integral ∫PdV because we have no way of assigning P to a system out of equilibrium.
False. Even if we can’t define the equilibrium pressure of the gas, we can still measure the external pressure on the piston as a function of volume, in principle. The work is the integral ∫Pext dV.
What is the relation between P and V for a reversible isothermal expansion of an ideal gas?
P = nRT/V
What is the relation between P and V for a reversible adiabatic expansion of an ideal gas?
P = const./V^γ where the exponent is γ = Cp/Cv
True or False? The energy of an ideal gas is given by the following expression U = Cv x T.
True. The energy of an ideal gas is a function of temperature only. Since the derivative of U with respect to T is equal to Cv, it follows that Cv is the proportionality constant between U and T.
What mathematical relation is regarded as a formal statement of the first law of thermodynamics?
∆U = Q −W
What convention do we follow? Is (a) Q the heat added to the system from the surroundings, or is (b) Q the heat passed to the surroundings from the system?
Q is the heat added to the system from the surroundings. Thus Q is positive if the heat is transferred to the system from the surroundings, and Q is negative if the heat is transferred from the system to the surroundings.
What convention do we follow? Is (a) W the work done on the system by the surroundings, or is (b) W the work done on the surroundings by the system?
Our convention is that W is the work done on the surroundings by the system. if the system does work on the surroundings, W is positive. If the surroundings do work on the system, W is negative.
In thermodynamics we talk about the system and the surroundings. What is the system? What is the surroundings?
The system is that part of the universe on which we are focusing our attention. The surroundings is the rest of the universe.
The first law is said to be a postulate consisting of two parts. What are these two parts?
The first part of the first law is our postulate that the energy of the universe never changes. The second part of the first law is postulate that energy U is a state function.
Reversible work is performed when the difference between the pressure of the system and the pressure of the surroundings is infinitesimal. What is the analogous requirement on temperature so that heat is transferred reversibly?
There should be an infinitesimal difference between the temperature of the system and the temperature of the surroundings in order to keep the system in equilibrium. (The surroundings always stays in equilibrium because of its vast size).
True or False? The state of a system is entirely determined by the specificatio of just two state variables.
True. For example, if you specify V and T for an ideal gas, then you can figure out P from the equation of state. You can also figure out I since U is Cv x T. (We are assuming that the number of moles of gas is known.)
For an ideal gas, find an expression for the following quantity: (∂P/∂T)v
nR/V
What is the heat capacity at constant volume for an ideal gas consisting of diatomic molecules? For example, dilute nitrogen or oxygen gas.
Cv =5/2 nR
What is the relation between the heat capacity at constant pressure and the heat capacity at constant volume for an ideal gas?
Cp = Cv + nR
True or False? It can be proved that the heat capacity at constant pressure is greater than the heat capacity at constant volume, regardless of the substance.
True.
The energy of a certain real (non-ideal) gas is given by U = 3/2 nRT - a n²/V. Calculate the coefficients of an expansion of U(T,V) in the variables T and V.
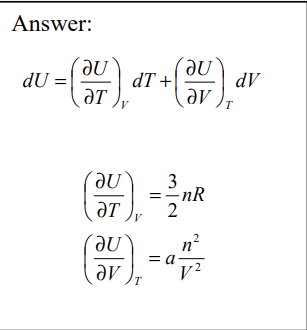
The energy of a certain real (non-ideal) gas is given by U = 3/2 nRT - a n²/V What is the heat capacity at constant volume?
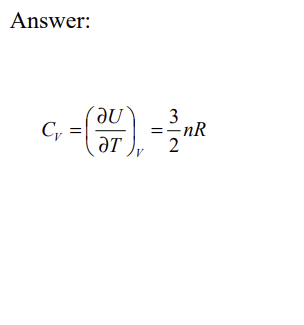
The energy of a certain real (non-ideal) gas is given by U = 3/2 nRT - a n²/V The equation of state for the gas is PV = nRT - an²/V Find the enthalpy H(T,V) as a function of T and V.

Who showed that the energy of an ideal gas depends only on its temperature? They also measured the dependence of U with V for real gases.
James Joule and William Thomson (later to be Lord Kelvin).
Who patented a heat engine in 1816, for the pupose of powering underwater torpedos without leaving a trail of bubbles?
Robert Stirling, 1816.
Who devised a hypothetical heat engine that could be run reversibly between two heat reservoirs, one reservoir kept at a hot temperature, the other at a cold temperature?
Sadi Carnot, 1820.
Who is credited with figuring out the second law of thermodynamics?
Rudolph Clausius, 1850.
Who investigated the properties of gases and found that at constant temperature, the pressure is inversely proportional to the volume?
Robert Boyle, 1662.
Who investigated the properties of gases and found that at constant pressure, the volume is proportional to the temperature?
Jacques Charles, 1787.
Why is it considered bad form to write dQ and dW when referring to small amounts of heat and small amounts of work?
In calculus, the differential “d” implies a change. Heat Q and work W are quantities that are exchanged between system and surroundings. While their exchange causes a change in U, it makes no sense to talk about a change in the quantity exchanged.
True or False? The entropy of S is a state function. It is sometimes related to heat, and sometimes not related to heat. It is related to heat when heat is transferred reversibly.
True.
True or False? The entropy of a system always increases when a process occurs spontaneously.
False. It is the sum of the entropy of the system plus the entropy of the surroundings that always increases for a spontaneous process, i.e. the entropy of the universe always increases.
What is the connection between an irreversible process and a spontaneous process?
All spontaneous processes, i.e. processes that occur in a finite amount of time, require an imbalance of forces (imbalance of pressure, or imbalance of temperature, etc.) They are therefore irreversible processes.
A system consists of an ice cube. It is initially at a temperature of 270 K and a pressure of 1 atm. It melts, becoming water at 294 K and a pressure of 1 atm. True or False? The change in entropy of the system depends on whether the melting takes place slowly or quickly.
False. Entropy is a state function, so the change in S only depends on the final and initial states. It does ont depend on the path taken to go from one state to the other.
True or False? The entropy of the universe always increases when a process occurs; it never decreases.
True. Entropy of the universe increases when a spontaneous process occurs, but spontaneous processes are the only processes that occur. Hence the entropy of the universe always increases.