CH14 LEC: CMPNDS W/ O, S, OR HALOGENS
1/198
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
199 Terms
disulfide originates from what rxn?
oxidation of 2 thiols
Alpha Carbon (Cα) definition
the C atom directly attached to a functional group of interest
alpha Cα always had ___ bonds
4 single
alcohol functional group definition
an organic compound w/ an —OH group usually linked to a saturated C atom (AKA alpha C)
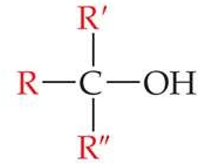
ID this structure
typical structure of an alcohol, where R, R’, R” can be H, alkyl group, or similar groups, alone or in combination
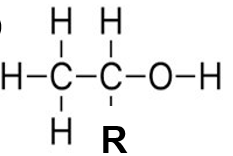
ID the family of this structure
alcohol
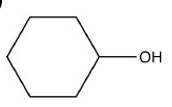
ID the family of this structure
alcohol (NOT phenol)
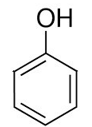
ID this structure
phenol — not classified as an alcohol!
no primary, secondary, or tertiary alcohols
aliphatic alcohols definition
organic compounds in which the —OH group is attached to an aliphatic C [straight-chain, branched, or non-aromatic ring structures]
alcohols are classified as ___, ___, and ___ according to the number of Cs substituents (alkyl or R-groups) bonded to the alpha C (—C—OH)
primary, secondary, tertiary
methanol’s (CH3OH) classification is considered ___ despite no alkyl group bond to the alkyl carbon
primary
phenol compound definition
alcoholic aromatic compound w/ an —OH group replacing 1 H atom in a benzene ring
benzene ring affects phenol’s ___ and ___, distinguishing it from aliphatic alcohols.
reactivity and properties
recognize this: C6H6
benzene
recognize this: C6H5OH or C6H6O
phenol
recognize this: —C—O—C— …or… —R—O—R—
ether
ether compound definition
any class of organic compounds that contains O b/t 2 alkyl or aryl groups (functional groups derived from aromatic ring)
aryl group definition
functional group derived from an aromatic ring
prefix in common naming of aliphatic alcohols: n—
indicates a straight chain
prefix in common naming of aliphatic alcohols: iso—
branch at second-to-last C (Y shape)
prefix in common naming of aliphatic alcohols: sec—
OH on a secondary C
prefix in common naming of aliphatic alcohols: tert—
OH on a tertiary C
prefix in common naming of aliphatic alcohols: neo—
C skeleton ends in quaternary C
naming common names of aliphatic alcohols: what do we add as the suffix?
“alcohol” — i.e., “alkyl alcohol”
naming common names of aliphatic alcohols: what number do we apply to the position of the —OH group?
the smallest number
naming alcohols — IUPAC names: alcohols are named by replacing the suffix “—ane” of alkanes w/ ___.
“—anol”
naming alcohols w/ alkyl substituent(s): the parent H—C chain includes the ___.
—OHs
naming alcohols w/ alkyl substituent(s): number the C atoms of the parent chain beginning at ___.
the end nearer the alcohol functional group — then the other substituent groups.
naming alcohols w/ alkyl substituent(s): add the location of the alcohol group by placing the number that locates the —OH group ___.
immediately before the parent-suffix.
naming alcohols w/ alkyl substituent(s): name and number the positions of the alkyl substituents___.
after you have numbered the —OH group
REMEMBER TO ALPHABETIZE 🙂
naming alcohols w/ alkyl substituent(s): for alcohols w/ 2 OH groups, name as ___.
alkanediol — keep the “e”.
naming alcohols w/ alkyl substituent(s): for alcohols w/ 3 OH groups, name as ___.
alkanetriol, and so on.
naming alcohols w/ alkyl substituent(s): the chain is numbered so as to give one of the —OH groups the ___.
lowest possible number
polyhydric alcohols definition
alcohols w/ 2 or 3 hydroxyl groups (—OH)
cyclic alcohols definition
alcohols w/ cyclic hydrocarbons chains
for cyclic alkanes and alkenes that contain an —OH group, name them as ___ and ___, respectively.
cycloalkanol and cycloalkenol
for cyclic alkanes/enes naming: the —OH is presumed to be bound to the ___ and proceeds in the direction that gives the other substituent(s) or functional groups the ___.
1st carbon — do not write!
lowest possible number
to name compounds w/ a phenol or one other substituent: the parent is ___ and we assign the C attached to ___ as C1.
phenol
—OH
to name compounds w/ a phenol or one other substituent: number the Cs in the ring so that the substituent ___.
get the smallest number.
to name compounds w/ a phenol or one other substituent: sometimes ___, ___, and ___ are used instead of numbers
o, m, and p
naming alcohols as substituent: named as a substituent when other functional group w/ a ___ is present.
higher ranking (such as aldehyde)
naming alcohols as substituent: as a substituent, the prefix for alcohol is ___.
“hydroxy”
lower rankings below alcohols are ___, ___, and ___.
alkyl groups, alkanes/ynes, and halogens
common names for methanol
methyl alcohol or wood alcohol
uses of methanol
antifreeze, solvent, fuel, and to denature ethanol
what does denaturing ethanol do
makes it undrinkable
how is methanol toxic
it is not directly harmful, rather when it is metabolized in the body, it converts into toxic compounds (methanal and methanoic acid)
15 mL leads to blindness
30 mL leads to death
recognize this: CH3OH
methanol
recognize this: CH3CH2OH …or… C2H5OH …or… C2H6O
ethanol
commons names for ethanol
ethyl alcohol or grain alcohol
why is ethanol called grain alcohol
b/c it can be fermented from grains by a process called alcohol fermentation in yeast - it is present in all alcoholic beverages
industrial ethanol production via ___
hydration of ethene in an addition rxn
ethene + water → ethanol
ethanol is treated with denaturants ___ or ___ to make it unfit for human consumption
methanol or isopropanol
industrial purpose of ethanol vs. beverage purpose of ethanol
ethanol used in beverages is subject to taxes, but when used in industrial purposes (cleaning products, fuels, solvents), these taxes can be avoided by adding denaturants to make it undrinkable
recognize this: C3H8O
2-propanol
common names for 2-propanol
isopropanol, isopryl alcohol, or rubbing alcohol
uses of 2-propanol
common solvent, used to sterilize instruments, and as a skin cleanser before drawing blood or giving injections
hand sanitizer contains 70—80% of ___.
ethanol
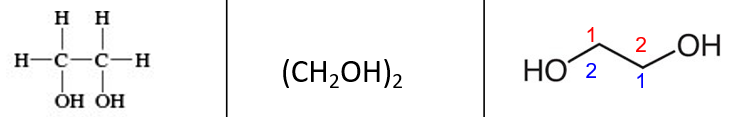
ID this structure: common and IUPAC name
common: ethylene glycol (EG)
IUPAC: 1,2-ethanediol
ethylene glycol does NOT contain ___.
C=C
characteristics of ethylene glycol
2 —OH groups
colorless liquid
miscible w/ H2O
sweet taste
lethal to humans and dogs
common use of ethylene glycol
antifreeze
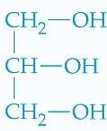
ID this structure: common and IUPAC name
common: glycerol or glycerin
IUPAC: 1,2,3-propanetriol
characteristics of glycerol
contains 3 Cs, each bound to an —OH group
nontoxic liquid
miscible w/ H2O
forms structural backbone of fats and oils
common use of glycerol
used as moisturizer b/c —OH groups interact w/ H2O
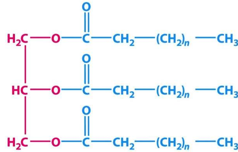
ID this structure
triacylglycerol
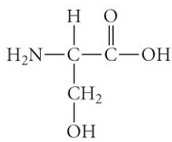
ID this structure
serine
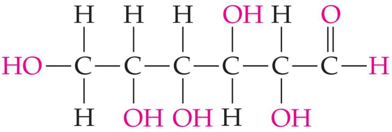
ID this structure
glucose
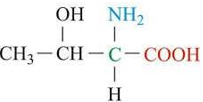
ID this structure
threonine
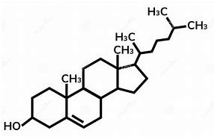
ID this structure
cholesterol
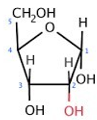
ID this structure
ribose
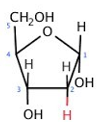
ID this structure
deoxyribose
2 kinds of alcohols
aliphatic and phenols
2 kinds of ethers
saturated (alkyl) and unsaturated (aryl)
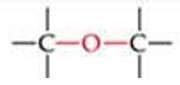
ID this structure
ether
aryl group definition
indicates aromatic functional group
Ar—OH
ethers can be present in (attached to or integrated in) these four forms
linear, cyclic, ring, or benzene rings
ether common naming: the prefix “di—” is used if ___.
both the alkyl groups are the same
ether common naming: ID the ___ groups attached in the O atom
2 alkyl
ether common naming: put the alkyl groups in ___ w/ spaces b/t the names and followed by the word ___.
alphabetical order
ether
ether IUPAC naming: ID ___ groups connected to O atom
alkane/alkyl
ether IUPAC naming: select the ___ alkyl group as the parent chain, and name it as an ___.
longer (if there is one)
alkane
ether IUPAC naming: name the shorter alkyl group and add the suffix ___ to indicate it is part of the ether
“—oxy”
ether IUPAC naming: number the C atoms in the parent chain to provide the lowest numbers for the ___.
O atom
ether IUPAC naming: ethers are typically names as ___ w/in a larger molecule(s)
substituents
ether IUPAC naming: the smaller, shorter alkyl group becomes the ___, and the larger alkyl group becomes the ___.
alkoxy substituent
alkane root name
ether IUPAC naming: in ROR’, R’ is larger than R and is named as ___.
alkoxyalkane
ether IUPAC naming: an —OR group is referred to as an ___.
alkoxy group
3 major types of intermolecular forces
London dispersion forces
Dipole-dipole
Hydrogen bonding
all molecules experience this force
london dispersion forces
london dispersion forces definition
at any given moment, there might be an unequal distribution of e- w/in a molecule, leading to a momentary polarity and then a temporary attractive force
LDFs strength
weak force
LDFs intensity influenced by what
size and structure of molecules
LDFs are the only intermolecular interactions for ___ molecules
nonpolar - i.e., hydrocarbons
larger the molecular weight and surface area, the ___ the forces, and ___ BP
greater
higher
BP ___ as alkanes increase in size
increase
branching ___ intermolecular interactions mainly b/c it ___ the surface area available for contact b/t molecules
decreases
decreases
BP ___ w/ ___ branching in isomers
decreases
increased
dipole-dipole forces definition
electrostatic attraction force b/t positive and negative ends of polar molecules
strength of dipole-dipole
stronger than LDFs and weaker than H-bonding