EXAM #2 STUDY SET
1/397
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
398 Terms
What is the functional group structure of an aromatic compound?
A six-carbon ring with alternating double bonds (benzene ring).
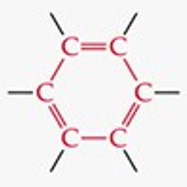
Give a simple example of an aromatic compound.
Benzene (C₆H₆).
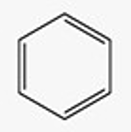
Recognize this line structure:
Aromatic compound.
What suffix is used for aromatics?
None.
What is the functional group structure of an alkane?
Contains only C–C and C–H single bonds; no readily reactive bonds.
Give a simple example of an alkane.
Propane (CH₃CH₂CH₃).
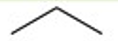
Recognize this line structure:
Alkane.
What is the suffix for alkanes?
-ane.
What is the functional group structure of an alkene?
Carbon–carbon double bond (C=C).
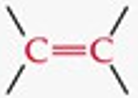
Give a simple example of an alkene.
Ethylene (H₂C=CH₂).
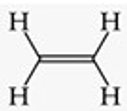
Recognize this line structure:
Alkene.
What is the suffix for alkenes?
-ene.
What is the functional group structure of an alkyne?
Carbon–carbon triple bond (C≡C).
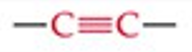
Give a simple example of an alkyne.
Acetylene (ethyne, HC≡CH).

Recognize this line structure:
Alkyne.
What is the suffix for alkynes?
-yne.
What is the functional group structure of an alkyl halide?
A carbon bonded to a halogen (F, Cl, Br, or I).
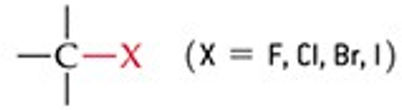
Give a simple example of an alkyl halide.
Ethyl chloride (CH₃CH₂Cl).
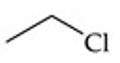
Recognize this line structure:
Alkyl halide.
What is the suffix for alkyl halides?
None.
What is the functional group structure of an alcohol?
Hydroxyl group (–OH).
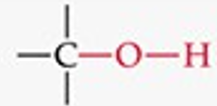
Give a simple example of an alcohol.
Ethanol (CH₃CH₂OH).
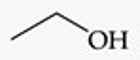
Recognize this line structure:
Alcohol.
What is the suffix for alcohols?
-ol.
What is the functional group structure of an ether?
Oxygen atom bonded to two carbons (R–O–R).
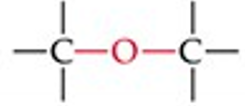
Give a simple example of an ether.
Diethyl ether (CH₃CH₂–O–CH₂CH₃).
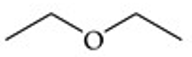
Recognize this line structure:
Ether.
What is the suffix for ethers?
None.
What is the functional group structure of an amine?
–NH₂ (amino group).
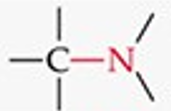
Give a simple example of an amine.
Ethylamine (CH₃CH₂NH₂).
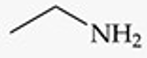
Recognize this line structure:
Amine.
What is the suffix for amines?
-amine.
What is the functional group structure of an aldehyde?
Carbonyl group (C=O) at the end of a chain, bonded to H (–CHO).
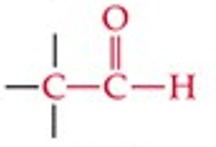
Give a simple example of an aldehyde.
Acetaldehyde (Ethanal, CH₃CHO).
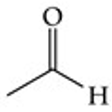
Recognize this line structure:
Aldehyde.
What is the suffix for aldehydes?
-al.
What is the functional group structure of a ketone?
Carbonyl group (C=O) bonded to two carbons.
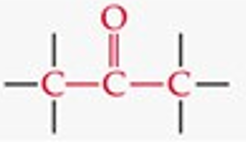
Give a simple example of a ketone.
Acetone (CH₃COCH₃).
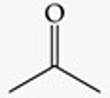
Recognize this line structure:
Ketone.
What is the suffix for ketones?
-one.
What is the functional group structure of a carboxylic acid?
–COOH (carbonyl + hydroxyl group).
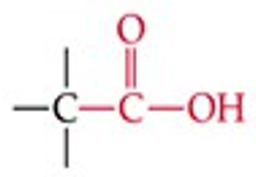
Give a simple example of a carboxylic acid.
Acetic acid (CH₃COOH).
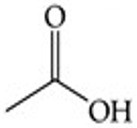
Recognize this line structure:
Carboxylic acid.
What is the suffix for carboxylic acids?
-ic acid.
What is the functional group structure of an anhydride?
Two carbonyls (C=O) bonded through an oxygen atom (–CO–O–CO–).
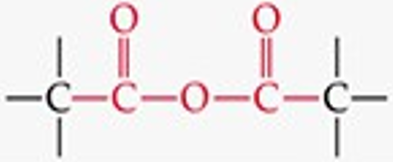
Give a simple example of an anhydride.
Acetic anhydride.
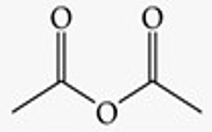
Recognize this line structure:
Anhydride.
What is the suffix for anhydrides?
None.
What is the functional group structure of an ester?
–COOR (carbonyl bonded to –OR group).
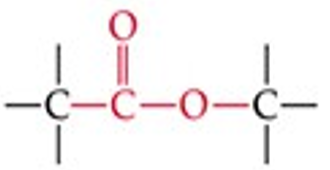
Give a simple example of an ester.
Methyl acetate (CH₃COOCH₃).
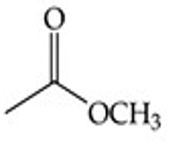
Recognize this line structure:
Ester.
What is the suffix for esters?
-ate.
What is the functional group structure of an amide?
–CONH₂ (carbonyl bonded to NH₂).
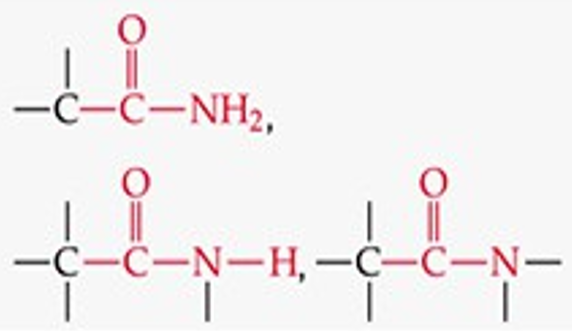
Give a simple example of an amide.
Acetamide (CH₃CONH₂).
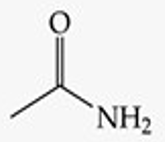
Recognize this line structure:
Amide.
What is the suffix for amides?
-amide.
What is the functional group structure of a thiol?
–SH (sulfhydryl group).
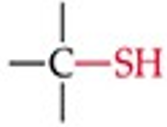
Give a simple example of a thiol.
Ethyl thiol (CH₃CH₂SH).
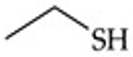
Recognize this line structure:
Thiol.
What is the suffix for thiols?
None.
What is the functional group structure of a sulfide?
R–S–R (sulfur atom bonded to two carbons).
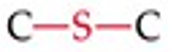
Give a simple example of a sulfide.
Ethyl methyl sulfide (CH3CH2SCH3)
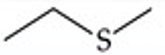
Recognize this line structure:
Sulfide.
What is the suffix for sulfides?
None.
What is the functional group structure of a disulfide?
R–S–S–R (two sulfur atoms bonded together, each bonded to carbon).

Give a simple example of a disulfide.
Dimethyl disulfide (CH₃–S–S–CH₃).
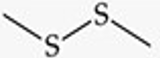
Recognize this line structure:
Disulfide.
What is the suffix for disulfides?
None.
ammonia structure
NH3
ammonium ion structure
NH4+
in ammonia, N atom as ___ v e-
5
in ammonia, ___ e- from N and ___ e- from ___ H atoms form ___ polar covalent bonds
3 (all spots)
ammonia is a ___ molecule
polar
in ammonia the remaining 2 e- (lone pair of nonbonding e-) can serve as ___ or ___.
H+ acceptor
e- donor
ammonia is a ___.
base
ammonium ion (cation) form a ___ w/ 4th H+ b/c H+ has ___ to form a bond.
coordinate bond
no e-
amine group is a functional group ___ from ammonia (NH3), in which 1 or more H atoms are replaced by ___.
derived
R-groups (alkyl or aryl)
amines can be classified as ___, ___, or ___, according to how many ___.
primary, secondary, tertiary
R-groups are individually bound directly to the N atom
primary amine definition
N atom is attached to only 1 R-group and 2 Hs
can be located on the end or attached to an internal C of a chain
primary amine general formula
R—NH2
secondary amine definition
N atom is attached to 2 R-groups and 1 H
secondary amine general formula
R2—NH
tertiary amine definition
N atom is attached to 3 R-groups and 0 Hs
tertiary amine general formula
R3—N
quaternary amine definition
N atom is attached to 4 R-groups and a (+) charge
quaternary amine general formula
R4—N+
amines are ___ b/c the N atom has a lone pair of e-, and this lone pair can accept a H+, leading to the formation of ___.
bases
an ammonium ion
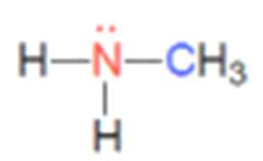
what class of amine is this
primary amine
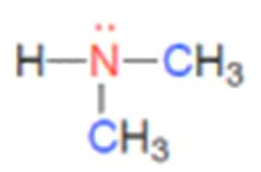
what class of amine is this
secondary amine
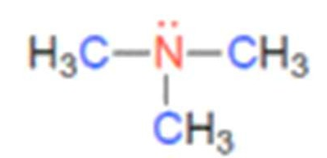
what class of amine is this
tertiary amine
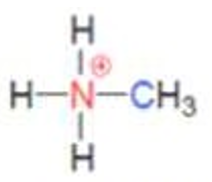
what class of ammonium ion is this
primary ammonium ion
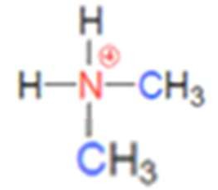
what class of ammonium ion is this
secondary ammonium ion
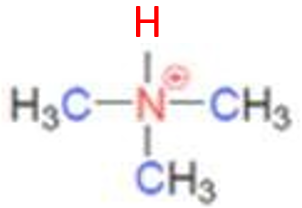
what class of ammonium ion is this
tertiary ammonium ion
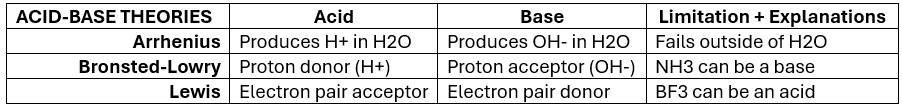
Acid-Base Theories — Summary Chart (KNOW)
nonpolar covalent bond’s electronegativity difference between atoms
0 — 0.4
polar covalent bond’s electronegativity difference between atoms
0.5 — 1.9
ionic bond’s electronegativity difference between atoms
2.0 and above
conjugate acid-base pairs definition
occur within acid-base equilibrium rxns
an acid and its conjugate base, as well as its base and conjugate acid, differ only by 1 proton.
HA + B ←→ A- + HB+
[acid + base ←→ conjugate base + conjugate acid]
amine + acid —> ___ + base
ammonium ion
3 major types of amines depends upon ___.
the structure of the backbone