Chemistry - Organic Chemistry: Reactivity
1/43
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
44 Terms
radical
chemical species that has an unpaired electron (the only requirement)
indicated by a dot on the chemical species
if radical is made of several atoms dot is on atom with the unpaired electron
eg. CH3
reactivity of radicals
unpaired electron of radical makes it highly reactive with high enthalpy
favourable for radicals to react with lower enthalpy by: taking an electron from other species (which creates another radical) or combining with another radical to form a covalent bond
high reactivity means they are typically not very long lasting
homolytic fission
breaking covalent bonds by each atom taking an electron from the bond to form two radicals
homolytic fission of halogens is the initiation step in a sequence of steps that form a chain reaction
types of homolytic fission
thermolytic (heat)
photolytic (UV light)
reactivity of alkanes
relatively stable/un reactive due to strengths of c-c and c-h bonds
electro-negativities of carbon and hydrogen bonds are almost the same in alkanes so molecule is non polar
no electron deficient or electron rich areas to attract electrophiles/nucleophiles
only react in combustion reactions and undergo substitution by radicals
free radical substitution of alkanes
atom substituted by halogen
UV needed due to un-reactive nature of alkanes
3 steps: initiation step (halogen broken by UV energy to form two radicals) propagation step (radicals create further radicals in chain reaction) termination step (two free radicals collide)
propagation step
progression of substitution reaction in a chain reaction
radicals are very reactive and attack un-reactive alkanes
C-H bond breaks homolytically
alkyl free radical produced
can attack other halogens to form halogeno-alkanes and regenerate halogen radical
termination step
two free radicals react together, forming single un-reactive molecule
multiple products possible
nucleophilie
electron-rich species that can donate a pair of electrons
nucleophilic substitution reaction
nucleophile attacks a carbon atom that carries partial positive charge, an atom with partial negative charge is replaced by the nucleophile
hydrolysis of haloalkanes
haloalkanes undergo nucleophilic substitution due to polar c-x bond
nucleophile is OH-
aqueous solution of NaOH or KOH with ethanol is used for OH
Halogen becomes leaving group
why are halogens good leaving groups
have relatively weak bonds with carbon
have high electronegativity so the bonded electrons are drawn to halogen making carbon partially positive and susceptible to nucleophilic attack
rate of reaction depends in type of halogen in haloalkane
C-F, C-Cl, C-Br, C-l
neutral nucleophiles
when nucleophile is neutral like water initial product is positive. positive molecule then de-protonates and loss an H+ , becoming neutral.
heterolytic fission
breaking covalent bond so more electro-negative atom takes both electrons
forming a electrophile and a nucleophile
opposite of this is a coordination bond
electrophilic addition
addition of electrophile to alkene double bond
area of high electron density so very susceptible to the attack
bond breaks forming single c-c bonds
eg. steam to form alcohol
why does the double bon react with electrophiles
since alkene contains pi bonds it is possible to break the weaker pi bonds and create stronger sigma bonds. therefore alkanes can undergo addition reactions and are considered more reactive.
addition of water
alkenes react with steam (300, 60 Atmospheres, sulphuric or phosphoric acid) and water adds across double bond (hydration) so alkene to alcohol
Lewis definitions
Lewis acid: lone pair acceptor (electrophile)
Lewis bace: lone pair donor (nucleophile)
Bronsted-Lowry definitions
Acid: can donate H+
Base: can accept H+
complex ion
central transition metal ion surrounded by ligands which are bonded by dative covalent bonds
different ligands
monodentase, bidentase, multidentase
charge on complex ions
sum of the oxidation states of all the species present, so if ligands are neutral: overall charge is the same as the oxidation state of metal ion
coordination number: number of coordination bonds to metal ion
bidentase ligands
can each form two coordinate bonds to central metal ion because each ligand has 2 atoms with lone pairs
nucleophilic substitution in halogenoalkanes
halogen replaced by a nucleophile
can occur in two ways: sn1 and sn2
sn1
in tertiary halogenoalkanes (carbon attached to halogen is also bonded to 3 alkyl groups)
1 because rate of reaction depends on concentration of 1 reactant
2 step equation
steps of sn1
c-x bond breaks heterolytically and halogen leaves as an x- ion. (slow step)
tertiary carbocation is attacked by nucleophile
so energy profile has two transition states and a carbocation intermediate (exothermic)
sn2
in primary halogenoalkanes c is bonded to 1 alkyl group
one step reaction
nucleophile donates electron to positive carbon to form new bond and at the same time bond breaks and halogen takes both electrons (heterolytic fission)
remember to draw transition state in mechanism
steric hindrance
halogen causes steric hindrance
nucleophile can only attack from opposite side of C-Br
so there is an inversion of configuration
factors affecting rate of nucleophilic substitution
nature of nucleophile
halogen
structure of halogenoalkane
nature of nucleophile
more negative charge means higher electron density and stronger nucleophile
when nucleophiles have the same charge, electronegativity of atom carrying the lone pair becomes the deciding factor
lower electronegativity means stronger nucleophile
class of halogenoalkane
tertiary: sn1 (most stable)
secondary: mixed
primary: sn2
electrophilic addition reactions
addition of electrophile to alkene double bond
eg. hydrogen, steam, hydrogen halides, halogens
addition of hydrogen halides
molecule is polar
h atom acts as an electrophile by accepting electrons from double bond in alkene
h-br breaks heterolytically
formation of highly reactive carbocation intermediate which reacts with bromine ion
addition of halogens
same as halides with one exception
halide have permanent dipole whereas halogens have temporary dipole induced by repulsion of halogens by high electron density in the double bond
addition of water
water is a weak electrophile so needs strong acid catalyst (H3O+)
2 step reaction
pi electrons in double bond are attracted to catalyst. heterolytic fission and carbocation is formed.
water acts as a nucleophile and donates a pair of electrons to the positive carbon atom. forming c-o. Equilibrium is established between positive product and deprotonated product (alcohol) and catalyst is regenerated
carbocations definition
positively charged carbon atoms with only 3 covalent bonds instead of 4
inductive effect
alkyl groups attached to positively charged carbon are electron donating groups
illustrated by arrowheads to show alkyl groups pushing electrons towards positive carbon, decreasing its charge
so charge is spread more in carbocation making it more stable
therefore tertiary carbocations are the most stable
Markovnikov’s rule
predicts outcomes of electrophilic addition
in an addition of HX to an alkene the halogen ends up bonded to the most substituted carbon atom
in an addition of interhalogen to alkene the most electronegative halogen ends up bounded to the most substituted carbon.
applies to unsymmetrical alkenes
Markovnikov addition favours formation of major product
mechanism of addition in unsymmetrical alkene
2 ways for electrophile to attach
break double bond and attach to least substituted carbon (the one bonded to the most other carbons) creating the most stable carbocation and thereby forming major product
break double bond and attach to most substituted carbon, creating least stable carbocation and forming minor product
reactions in benzene
benzene undergoes a wide range of reactions including combustion and nitration
nitration involves substitution of a hydrogen atom from the benzene ring with an electrophilic atom/group of atoms
steps of electrophilic substition
generation of an electrophile
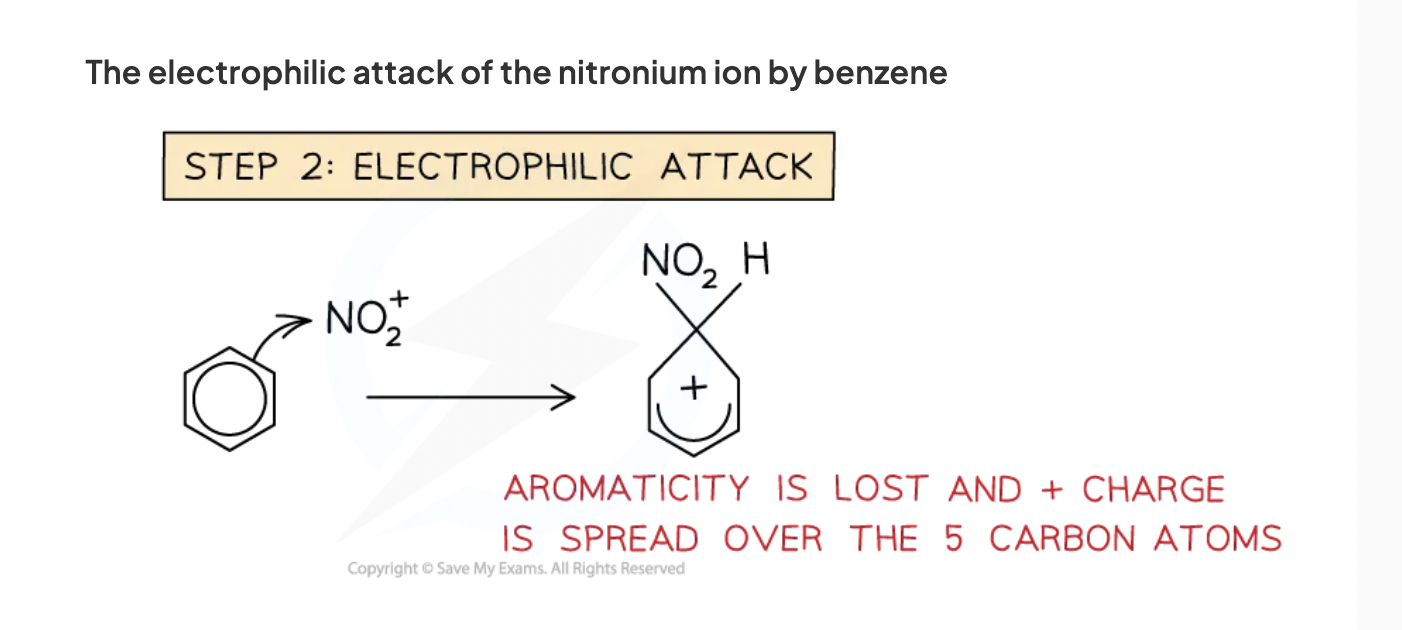
electrophile attack
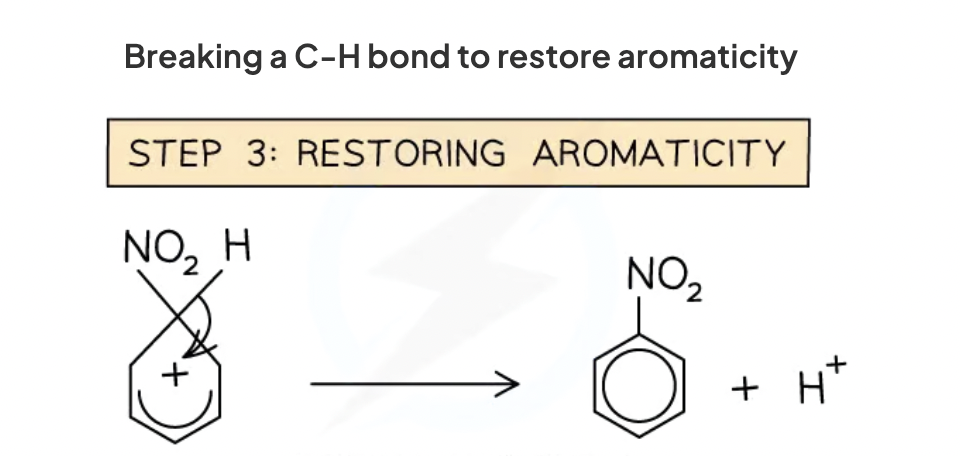
regenerating aromaticity
generation of an electrophile
delocalised pi system is stable and there is an increase in electron density
so first step is generating electrophile
in nitration that is nitronium ion no2+ produced in situ by adding concentration nitric and sulfuric acid to between 25-60 degrees into the mixture
electrophile attack
pair of electrons from benzene are donated to electrophile as covalent bond
now only 4 pi electrons and a positive charge is spread over carbons so aromaticity is lost.
regenerating aromaticity
heterolytic cleavage of c-h bond. so electrons in the bond go back to pi bond system.