7: Sugars & Glycobiology
5.0(1)
Card Sorting
1/41
Earn XP
Description and Tags
Last updated 10:25 PM on 11/15/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
42 Terms
1
New cards
What are the two families of monosaccharides?
Aldoses and Ketoses
2
New cards
Describe the basic structure of a common monosaccharide backbone. Differentiate aldose vs ketose.
Each consecutive connecting carbon within the backbone contains a hydroxyl group. There is also a carbonyl group present as well.
An aldose (ald-ehyde) has the carbonyl at the end (top) of the Fischer projection.
A ketose (ket-one) has the carbonyl sandwiched between backbone carbons.
An aldose (ald-ehyde) has the carbonyl at the end (top) of the Fischer projection.
A ketose (ket-one) has the carbonyl sandwiched between backbone carbons.
3
New cards
Describe key characteristics of a monosaccharide. (solubility, functional groups, number of carbons)
Highly soluble.
Polyhydroxyl aldehydes/ketones
3-7 carbons
Polyhydroxyl aldehydes/ketones
3-7 carbons
4
New cards
How do you differentiate a D vs L oriented sugar?
Using a Fischer projection, select the carbon furthest from the carbonyl to be the reference carbon. Then, observe the position of that carbon's hydroxyl relative to the backbone.
L: if the -OH of the reference carbon is to the left.
D: if the -OH of the reference carbon is to the right.
L: if the -OH of the reference carbon is to the left.
D: if the -OH of the reference carbon is to the right.
5
New cards
What are epimers?
Epimers are two sugars that differ only in configuration about one carbon.
Note: they may have differing names, as the difference in configuration may not be on the reference carbon. Therefore, it is not reliable to delineate epimers from D/L distinctions.
Note: they may have differing names, as the difference in configuration may not be on the reference carbon. Therefore, it is not reliable to delineate epimers from D/L distinctions.
6
New cards
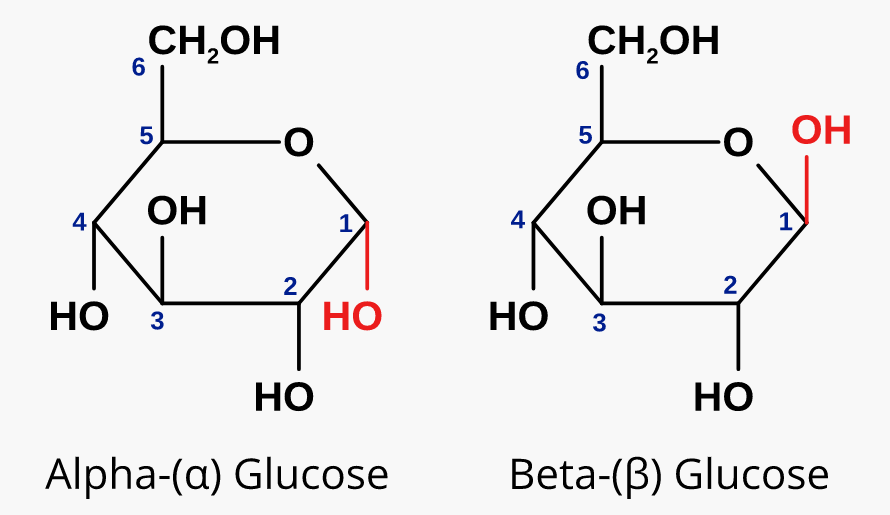
How do you differentiate between an α and β oriented sugar?
Relative to the CH2-OH group,
α: the C1 -OH group is OPPOSITE SIDE
β: the C1 -OH group is SAME SIDE
α: the C1 -OH group is OPPOSITE SIDE
β: the C1 -OH group is SAME SIDE
7
New cards
What are anomers?
Isomeric monosaccharides varying only in configuration about hemiacetal or hemiketal carbon. Makes more sense if you draw a Fischer projection and Haworth projection for a sugar.
Anomers are sugars that vary only in α/β configuration.
Anomers are sugars that vary only in α/β configuration.
8
New cards
What is a pyranose? What is a furanose? Are they planar?
Pyranose: 6-membered sugar ring
Furanose: 5-membered sugar ring.
Neither of these are planar.
Furanose: 5-membered sugar ring.
Neither of these are planar.
9
New cards
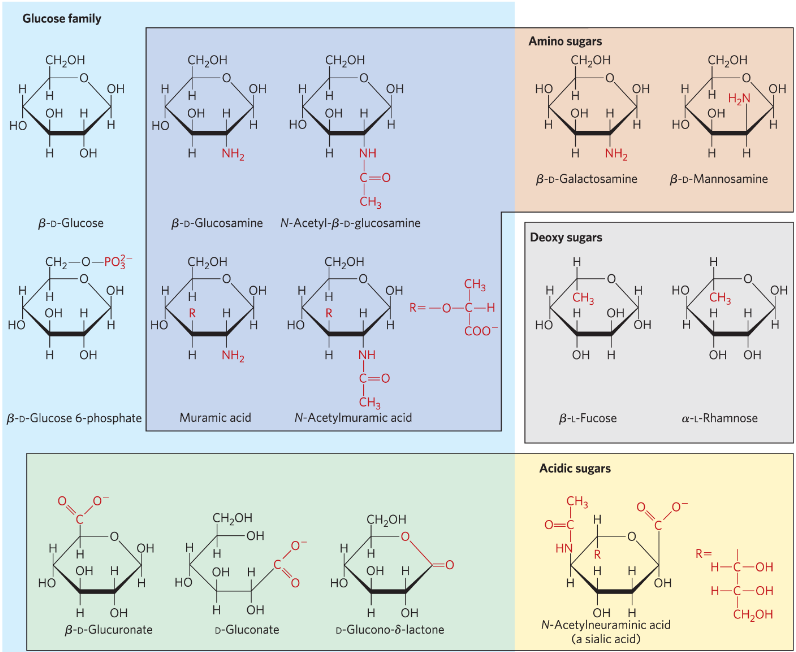
What are a few classes of hexose derivatives?
Amine-substituted sugars
Deoxy-sugars
Acidic-sugars
Sugar-phosphates
Deoxy-sugars
Acidic-sugars
Sugar-phosphates
10
New cards
What is a reducing sugar?
A sugar able to have its aldehyde undergo redox with a Cu2+ ion, under basic conditions. The sugar is oxidized into a carboxylic acid.
Reduces the Cu2+ to Cu+
Reduces the Cu2+ to Cu+
11
New cards
What is the key bond within a disaccharide?
An O-glycosidic bond links to monosaccharides into a disaccharide.
12
New cards
Glycosidic bonds are readily hydrolyzed by what?
Glycosidic bonds resist cleavage by what?
Glycosidic bonds resist cleavage by what?
Readily hydrolyzed by acid.
Resist cleavage by base.
Resist cleavage by base.
13
New cards
Describe interconversion of conformation by sugars.
Sugars usually can easily interconvert between linear and cyclic forms, or between chair conformations. However, within disaccharides and bigger sugars, the O-glycosidic bond prevents this interconversion from happening.
14
New cards
What common disaccharide do plants use to store sugar?
Sucrose
15
New cards
What are 4 ways that polysaccharides differ?
Monosaccharide component sequence
Glycosidic linkages
Chain length
Degree of branching
Glycosidic linkages
Chain length
Degree of branching
16
New cards
What are the functions of common polysaccharides in plants and animals and what are their names?
Plants: chitin, cellulose, used for structure; starch used as energy storage
Animals: glycogen, used as energy storage
Animals: glycogen, used as energy storage
17
New cards
What monosaccharide(s) make up starch? Describe their branching and linkages
Amylose: unbranched glucose polymer with α(1-4) linkages
Amylopectin: highly branched glucose polymer, with α(1-6) branch point linkages.
Amylopectin: highly branched glucose polymer, with α(1-6) branch point linkages.
18
New cards
What monosaccharide(s) make up glycogen?
Glucose, α(1-4) linkages, highly branched, more compact than starch.
19
New cards
A glycogen molecule with n number of branches has how many non-reducing ends?
n+1
20
New cards
How to determine if a sugar is reducing or not?
Reducing sugars have hemiacetals.
Nonreducing sugars have acetals that are "locked" within the ring and are thereby inactive.
Nonreducing sugars have acetals that are "locked" within the ring and are thereby inactive.
21
New cards
What is cellulose?
A homopolysaccharide that makes up cell walls of plants. Linear and unbranched, its key difference from amylose is that cellulose has β(1-4) linkages.
22
New cards
What is the most stable 3D structure for α(1-4) linkages, such as those present in starch and glycogen?
A helix, stabilized by inter-chain H-bonding
23
New cards
What is the most stable 3D structure for β(1-4) linkages, such as those present in cellulose?
A straight, extended chain, stabilized by intrachain and interchain hydrogen bonding. (They exist in flat planes of many chains)
Kinda like b sheets
Kinda like b sheets
24
New cards
What is peptidoglycan?
A heteropolysaccharide comprised of β(1-4) linked N-acetylglucosamine and N-acetylmuramic acid.
Makes up bacterial cell walls and creates stability in structure.
Makes up bacterial cell walls and creates stability in structure.
25
New cards
What is a glycosaminoglycan? Where is it found, and what are its components? Talk about its charge.
A family of linear polysaccharides found in animals and bacteria, but not plants. It is made up of repeating disaccharide subunits, typically comprised of N-acetyglucosamine and a uronic acid.
It has a high density of negative charge.
It has a high density of negative charge.
26
New cards
What is a glycoconjugate?
An information-carrying carbohydrate (sugar) that is covalently linked to either a protein or lipid.
27
New cards
What is a proteoglycan?
A proteoglycan is a glucosaminoglycan chain that is covalently linked to a core protein via a serine residue.
28
New cards
How does the glucosaminoglycan chain attach to the core protein?
It attaches via a tetrasaccharide linker.
29
New cards
Some proteoglycans are membrane proteins. What are the two main types?
Syndecans and glypicans
30
New cards
Describe the general function of the surface membrane syndecans and glypicans.
These proteins can be "shed" from the cell's membrane surface; the cell's surface features plays a role in cell-to-cell communication, recognition, adhesion, proliferation, and differentiation.
31
New cards
Describe proteoglycan aggregates.
Form from many core proteins that attach to a single long strand of hyaluronan. Have multiple chains of sulfates.
Contribute strongly to tensile strength and resilience of connective tissue.
Contribute strongly to tensile strength and resilience of connective tissue.
32
New cards
What is a glycoprotein? What two types of linkages can occur between the carbohydrate and the protein and which residues are involved?
A glycoprotein is a protein that has multiple oligosaccharides covalently bound. The glycans are branched and very structurally diverse.
N-linkages involve asparagine residues covalently linked to an oligosaccharide.
O-linkages involve serine or threonine residues covalently linked to an oligosaccharide.
N-linkages involve asparagine residues covalently linked to an oligosaccharide.
O-linkages involve serine or threonine residues covalently linked to an oligosaccharide.
33
New cards
About what percent of all mammalian proteins are glycosylated?
50%
34
New cards
What are glycolipids?
Glycolipids are components of the plasma membrane that have oligosaccharides as the hydrophobic head component.
35
New cards
What are mucins?
It is a type of glycoprotein containing many o-glycosidic bonds. They are present in most bodily secretions (i.e. mucus)
36
New cards
Give 3 examples of glycoproteins.
Antibodies, certain hormones, mucins.
37
New cards
What is a ganglioside?
Eukaryotic membrane lipids containing a polar head group composed of a complex oligosaccharide with a sialic acid.
38
New cards
What are lipopolysaccharides?
Sugar-lipid membrane molecules that interact with antibodies.
39
New cards
Why are oligosaccharides so information dense?
Compared with amino acids and nucleic acids, there are several orders of magnitude more of different permutations of a given amount of oligosaccharide subunits than of amino acid sequences or nucleic acid sequences of the same amount of subunits.
This is due to the prevalence of branched structures, nonexistent in nucleic or amino acids. Further, oligosaccharides have many more bond types and therefore variations of bound groups.
This is due to the prevalence of branched structures, nonexistent in nucleic or amino acids. Further, oligosaccharides have many more bond types and therefore variations of bound groups.
40
New cards
What is a lectin? What is its function?
Lectins are molecules essential to cell signaling. They are able to separate and detect glycans and glycoproteins with different oligosaccharide groups.
41
New cards
What is a selectin? What is its function?
Selectins are lectins specific to the plasma membrane. They commonly mediate the movement of immune cells to infection sites.
They are the main players of the inflammatory response.
This is done via the interaction of selectins with surface oligosaccharides on the leukocytes; this slows the leukocytes to allow them to bind to the infection site.
The second step is where integrin actually stops the leukocyte and mediates its cross over the capillary wall and to the infection site.
This process is known as "lymphocyte homing"
They are the main players of the inflammatory response.
This is done via the interaction of selectins with surface oligosaccharides on the leukocytes; this slows the leukocytes to allow them to bind to the infection site.
The second step is where integrin actually stops the leukocyte and mediates its cross over the capillary wall and to the infection site.
This process is known as "lymphocyte homing"
42
New cards
What is E-selectin and L-selectin's functions?
E-selectin: Binds endothelial oligosaccharide
L-selectin: Binds leukocyte oligosaccharide
Both during the process of lymphocyte homing.
L-selectin: Binds leukocyte oligosaccharide
Both during the process of lymphocyte homing.