Cartões: 5.14-5.16 Absolute and Relative Configuration; Physical Properties of Diastereomers; Resolution of Enantiomers | Quizlet
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
absolute configuration
the detailed stereochemical picture of a molecule, including how the atoms are arranged in space
relative configuration
the experimentally determined relationship between the configuration of two molecules, even though the absolute configuration of either may not be known
What can we do, experimentally, to determine that a product has the same relative configuration as the reactant?
use a reaction that does not break bonds at the asymmetric carbon atom(s)
D-L system
(Fischer-Rosanoff convention) D has the same relative configuration as (+)-glyceraldehyde. L has the same configuration as (-)-glyceraldehyde
The name (1S, 2R)-1-bromo-2-methylcyclohexane specifies the ____ configuration of one of the cis enantiomers.
absolute
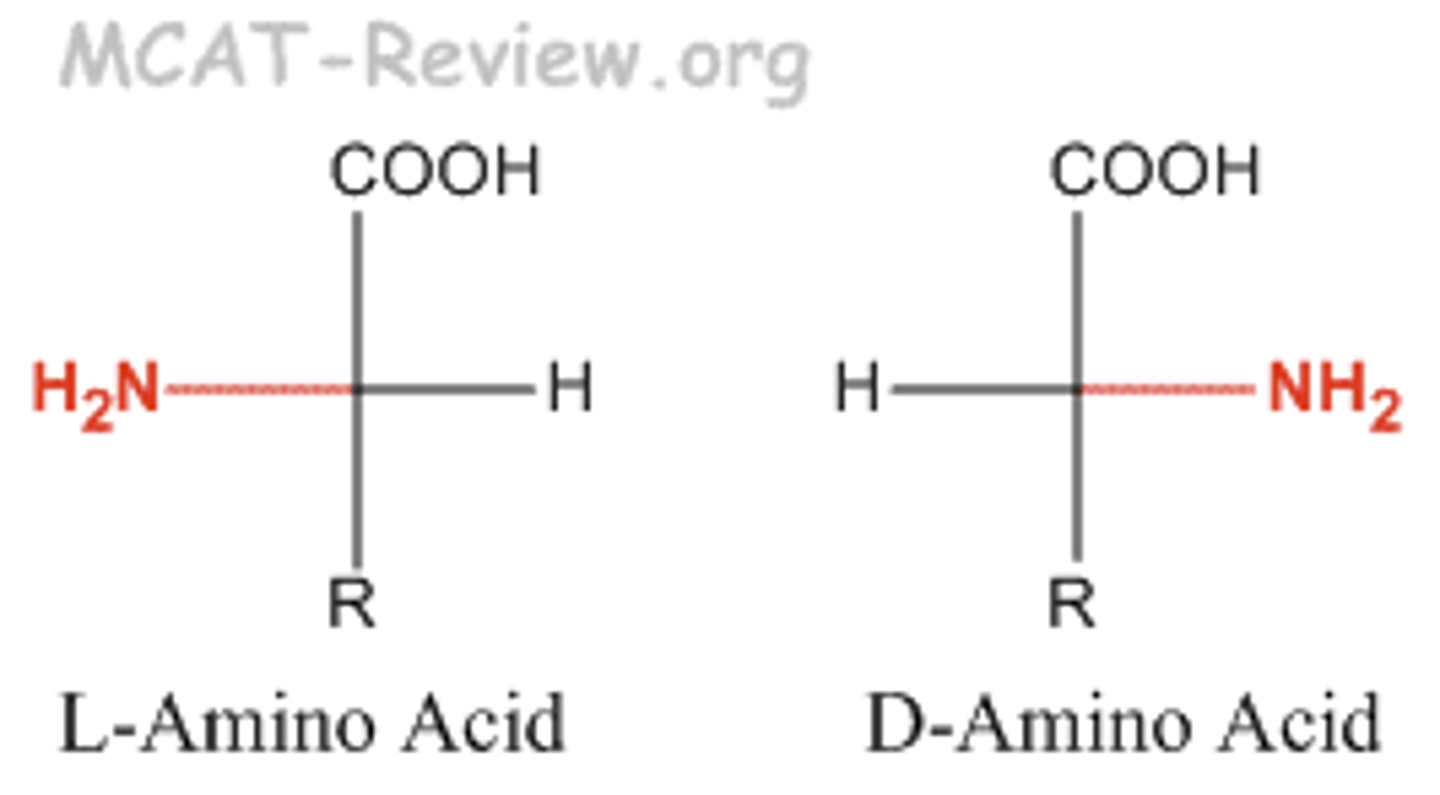
Most naturally occurring amino acids have the ____ configuration, with the ____ group on the left in the Fischer projection.
l; amino
Sugars have several asymmetric carbons, but they can all be degraded to ____ by oxidizing them from the ___ end.
glyceraldehyde; aldehyde
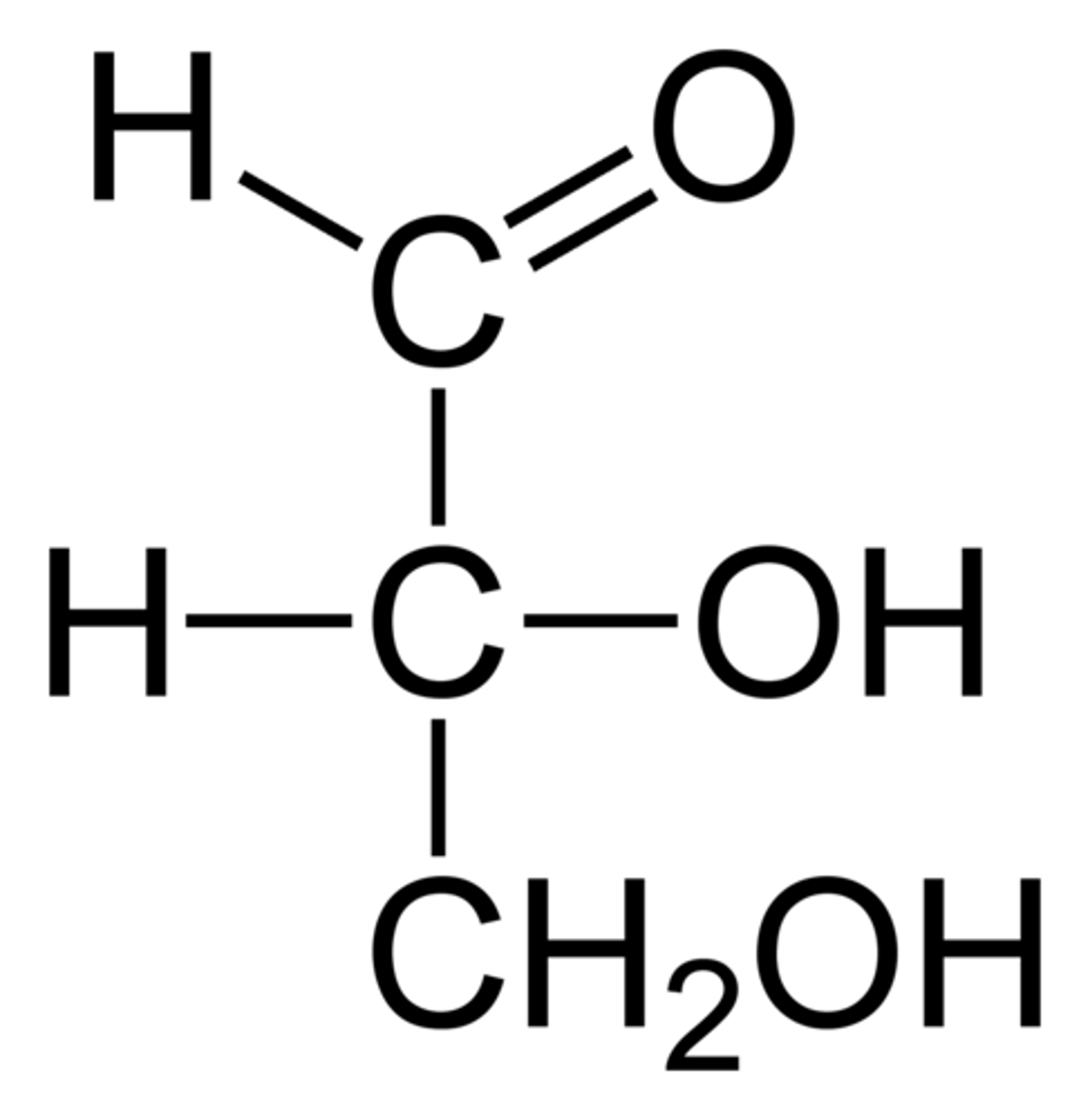
Most of the common sugars are ____ of glucose.
diastereomers
Why can we separate the diastereomers more easily than enantiomers?
Because diastereomers have different physical properties (so separation techniques will work)
Pure enantiomers of optically active compounds are often obtained by...
isolation from biological sources.
Most optically active molecules are found as only one enantiomer in living organisms.
true
What do you get when a chiral compound is synthesized from achiral reagents?
a racemic mixture of enantiomers
resolution
the process of separating a racemic mixture into a pure enantiomer
What is required to obtain resolution of an enantiomer from a racemic mixture?
a chiral resolving agent
resolving agent
a chiral compound (or chiral material on a chromatographic column) used for separating enantiomers
The traditional method for resolving a racemic mixture into its enantiomers is to use...
an enantiomerically pure natural product (resolving agent) that bonds with the compound to be resolved.
Chromatography is a powerful method for...
separating compounds.
____ can also be used to eliminate an undesired stereoisomer when trying to separate enantiomers.
Enzymes