26.1 The discovery of the nucleus
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
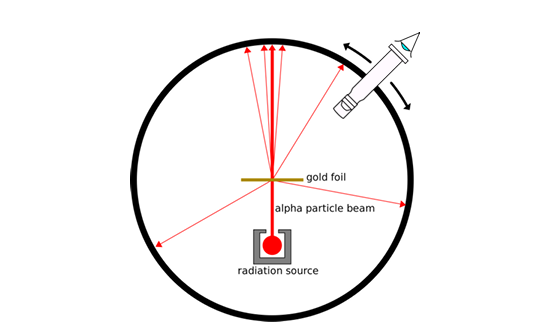
What were the observations made in the gold foil experiment?
Most alpha particles passed through the foil without deflection
1 in 2000 were deflected by small angles
About 1 in 10, 000 were deflected by angles greater than 90
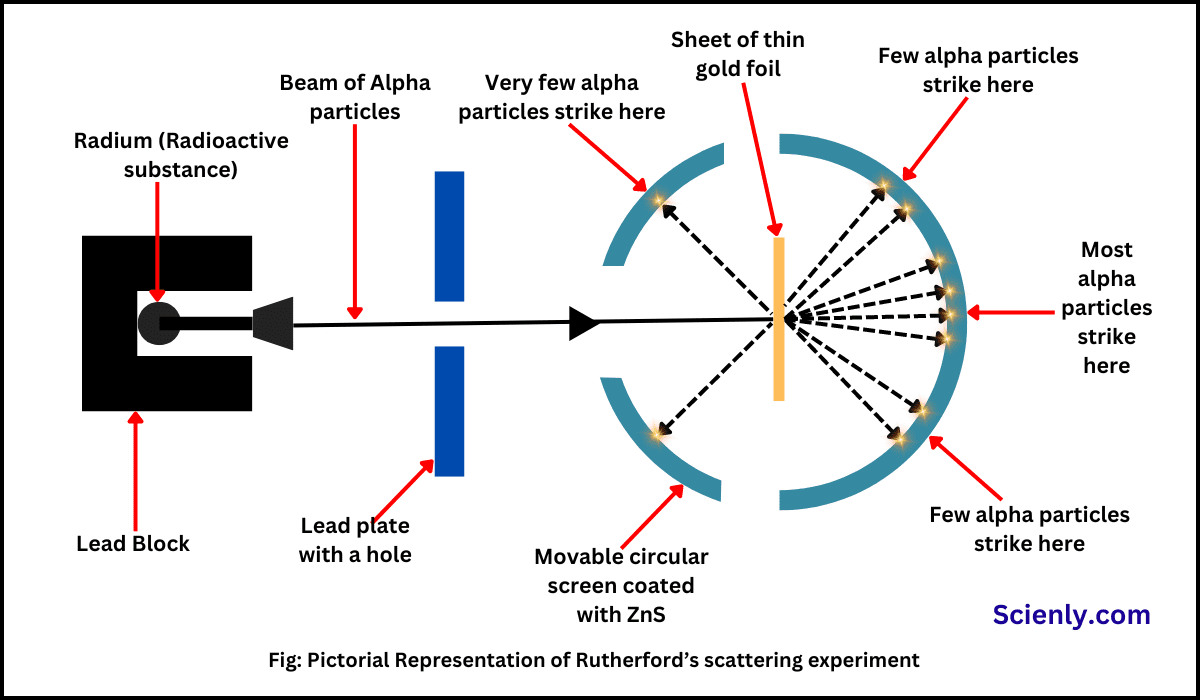
What did Rutherford’s gold foil experiment entail?
alpha beams were directed at a thin gold foil
in an evacuated chamber covered in fluorescent coating
moveable microscope
What was concluded from the observation that most alpha particles passed through the foil without deflection?
most of the atom is empty space
What was concluded from the observation that 1 in 2000 were deflected by small angles?
the atom has a small, dense positively charged nucleus
What was concluded from the observation that about 1 in 10, 000 were deflected by angles greater than 90?
most of the mass is concentrated in the centre
The closer the alpha particles approaches the nucleus,
the smaller the deflection
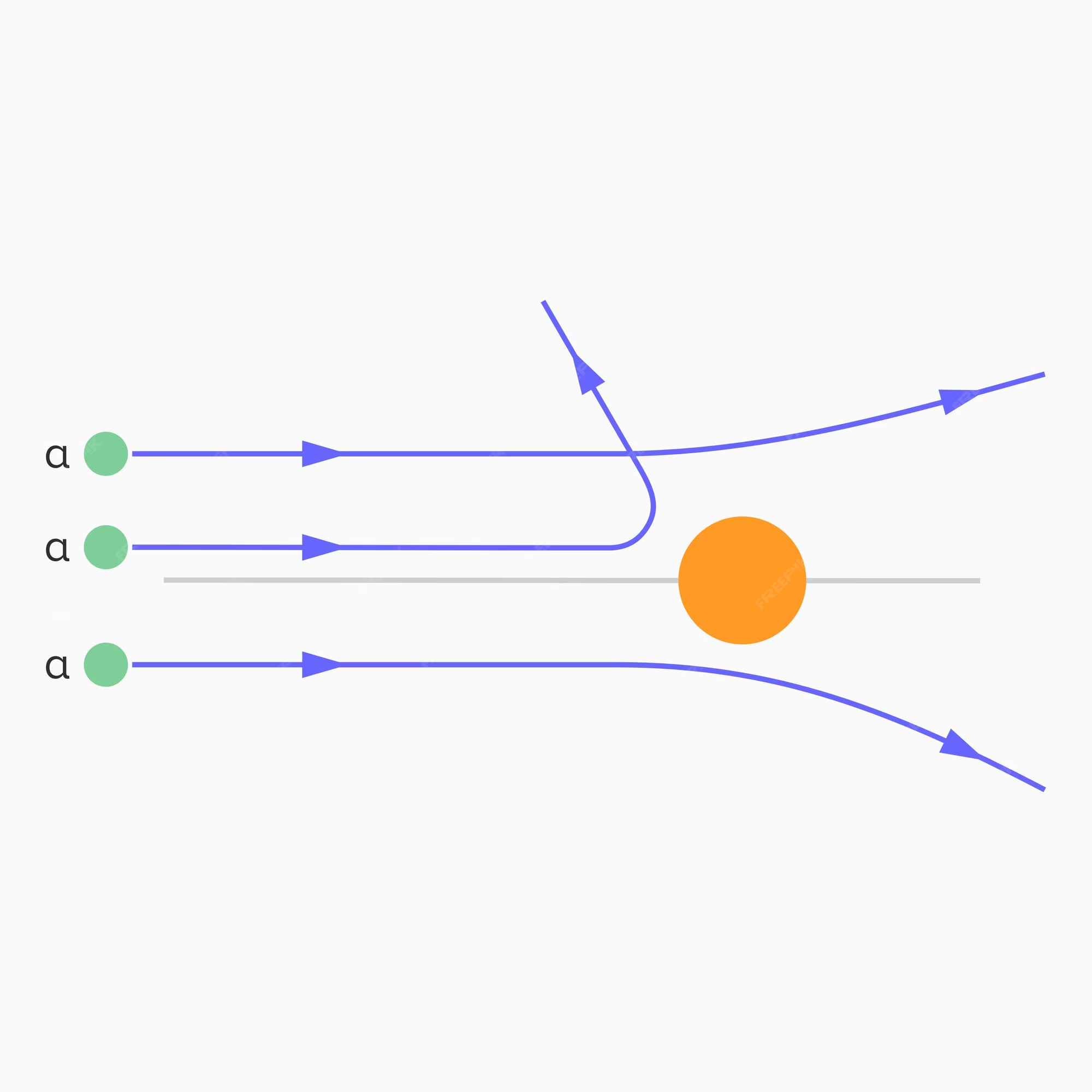
How can you calculate the size of the nucleus relative to the size of the atom?
