AP Chemistry Unit 1
1/35
Earn XP
Description and Tags
7%-9% Exam Weighting
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
The term is generic. We could be talking about an atom, molecule or formula unit.
They cannot really be counted or measured directly.
Chemists use them to connect the mass of substance reacting and the number of particles undergoing chemical changes.
1 of it = 6.022 × 10²³ particles
The formula unit is n
The number of particles of a substance in 1 mole of the substance
6.022 × 10²³
It provides a connection between the number of moles in a pure sample of a substance and the number of constituent particles (formula units) of that substance.
It (6.022 x 10²³) particles/mole is the conversion factor to convert between the number of particles (molecules, atoms, formula units, ions) and moles.
The mass of one mole of any element is equal to its relative atomic mass in grams.
E.g. one mole of Cl has a mass of 35.5 g and one mole of S has a mass of 32.1g
The atomic mass (or formula mass) in amu of a substance is numerically equivalent to the mass in g of one mole of that substance (the molar mass).
Molar Mass
It is the mass of one mole of a substance (measured in g/mol).
It (g/mol) is used as a conversion factor to convert between moles and grams.
Formula Unit
A set of an ionic compound. Eg. 3 formula units of PbI2 contain 3 Pb3+ ions and 6 I- ions.
It is unit of an ionic compound
Atoms and sub-atomic particles have a tiny mass. So we compare them by using _______
Relative masses
How do the masses of protons and neutrons relate?
They have virtually the same mass
How do the masses of electrons and protons relate?
An electron’s mass is negligible because it is so small
It weights around 1/1836th of a proton
Neutron
No charge
Located in nucleus
Relative mass = 1
Actual mass = 1.7 x 10^(-24)g
Proton
Positive charge
Located in nucleus
Relative mass = 1
Actual mass = 1.7 x 10^(-24)g
Electron
Negative charge
Located around nucleus
Relative mass = 0
Actual mass = 9.1 x 10^(-28)g
Mass number
The sum of the relative masses of protons and neutrons in and element
Isotopes
Atoms of the same element can have different numbers of neutrons, these are called isotopes.
Mass spectroscopy
Mass spectroscopy is a technique used to identify the isotopes of an element and their relative abundance in nature.
The mass spectrum of a sample containing a single element can be used to determine:
the identity of the isotopes of that element.
the relative abundance of each isotope in nature.
How does a mass spectrometer work?
The relative abundance of isotopes are found experimentally using a mass spectrometer:
The element sample is vaporised and ionised to form positive ions.
The ions are accelerated.
Heavier ions move more slowly and are deflected less, so the ions of isotopes are separated.
The ions are detected on a mass-to-charge ratio (m/z).
The greater the abundance, the more ions reach the detector.
Average atomic mass
Average atomic mass is the weighted mean mass of an element.
The weighted average takes into account the percentage abundance and the relative isotopic mass of each isotope.
(T or F) Most elements have only one isotope
False. Most elements are a mixture of isotopes, each with a different relative isotopic mass.
Steps to calculate average atomic mass of Cl:
Identify isotopes’ masses and % abundances from the graph
→35 and 75%, 37 and 25%
Multiply each isotope’s mass by its relative abundance (not % abundance)
→(35) (0.75) + (37) (0.25)
Add the results
→(26.25) + (9.25) = 35.5
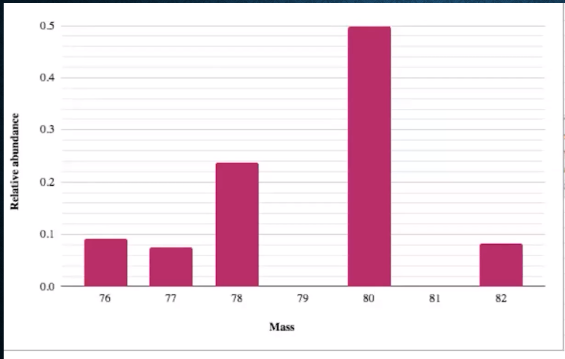
AP Question: What is the identity of this element?
Selenium
How do you estimate average atomic mass?
The average atomic mass of an element can be estimated using the mass of each isotope and nits relative abundance.
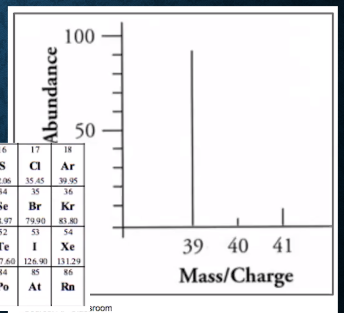
E.g., Identify the element shown in this mass spectrum graph.
39 u (about 90% abundance)
40 u and 41 u (together about 10% abundance)
The average atomic mass is likely to be slightly larger than 39.→The element is potassium
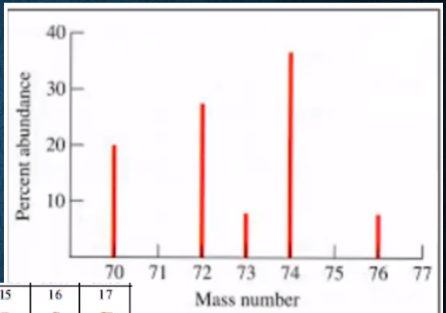
AP Question: Identify the element and number of neutrons in atoms represented by the peak at 74 amu in the mass spectrum.
Estimate mass→Mass ~72 – 74
Check periodic table→Ge = 72.63
Neutrons?→74-32 = 42 neutrons
The element is germanium
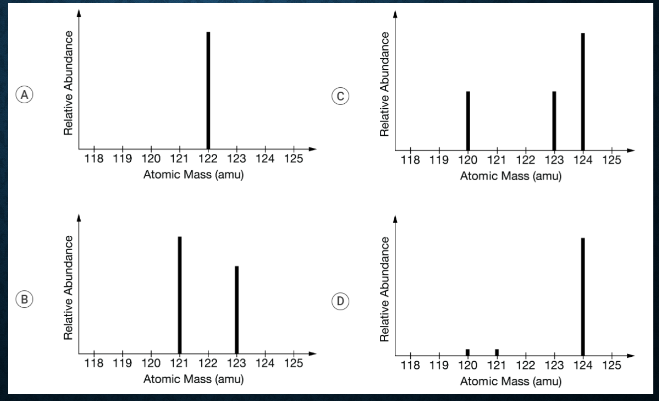
The mass spectrum of the element Sb is most likely represented by which of the following?
B
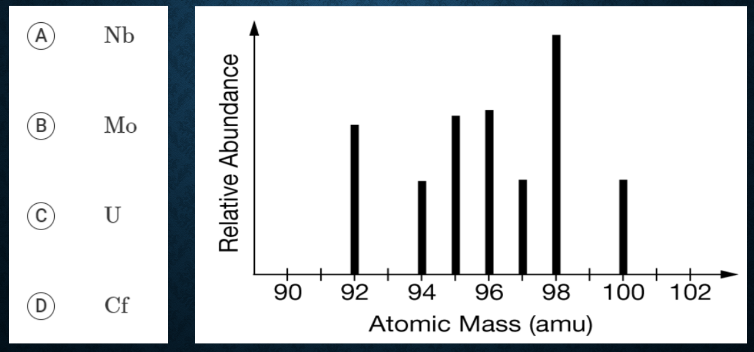
Which of the following elements has the mass spectrum represented below?
B
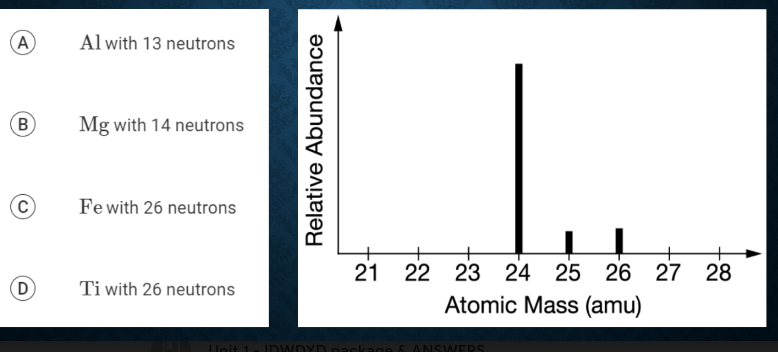
The mass spectrum of a sample of a pure element is shown above. Based on the data, the peak at 26amu represents an isotope of which of the following elements?
B
How to calculate % abundance
% abundance can be calculated from the average atomic mass and individual isotopic masses.
Remember that % abundance must always equal 100.
This value becomes 1 when considering relative abundance.
Steps:
Assume one isotope has an abundance of x.
The second isotope must have an abundance of 1-x.

10B = x & 11B = 1-x
10.8 = (10)(x) + (11)(1-x)
x = 0.2 and 1-x = 0.8
Therefore, C is the correct answer.