Synapses and Chemical neurotransmission
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
51 Terms
The neuronal synapse
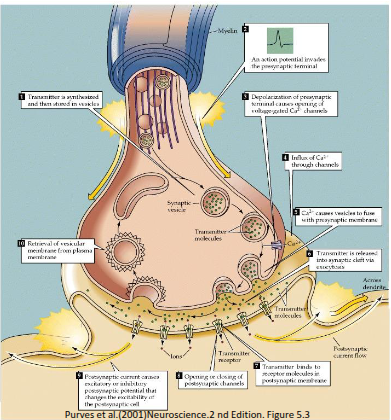
What are the key roles of ligand-gated receptors, voltage-gated ion channels, and gap junctions in signal transduction?
Ligand-gated receptors, such as those activated by acetylcholine, allow communication of nerve impulses between neurons.
Voltage-gated sodium and potassium channels propagate nerve impulses along the axon.
Gap junctions enable rapid, synchronised signalling between cells and permit the transfer of small metabolic nutrients.
What are voltage-gated channel receptors, and how do Na⁺ and K⁺ channels differ in structure?
Voltage-gated channels open in response to changes in membrane potential and include channels selective for Na⁺, Ca²⁺, K⁺ and Cl⁻. The Na⁺ channel consists of four subunits fused into a single polypeptide, whereas the K⁺ channel is formed from four separate subunits that assemble together.

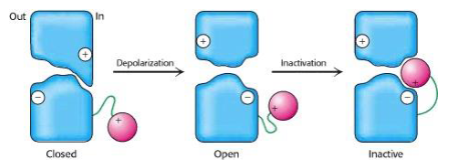
How does the ball-and-chain model explain voltage-gated channel inactivation?
In the ball-and-chain model, a positively charged “ball” attached to the channel by a flexible “chain” swings into a negatively charged binding site created upon depolarisation. This blocks the open channel, thereby inactivating it.
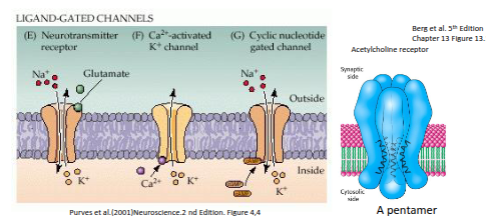
What activates ligand-gated channel receptors, and how does the acetylcholine receptor function?
Ligand-gated channel receptors are opened by extracellular neurotransmitters such as glutamate, or by intracellular second messengers like Ca²⁺, cAMP, or cGMP. The acetylcholine receptor is a pentamer, and ligand binding induces a structural change that opens the ion pore.
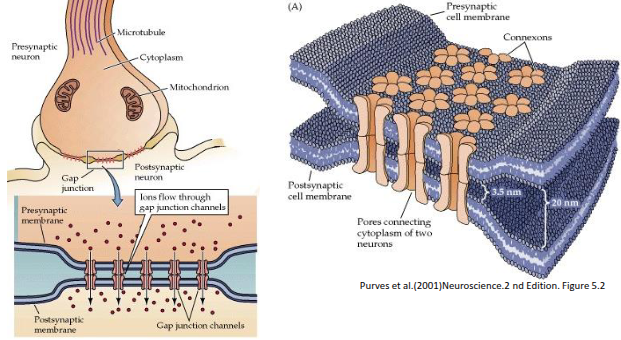
What are gap junctions, and what factors cause them to close?
Gap junctions are intercellular channels that allow direct electrical and metabolic communication between neighbouring cells. Once formed, they remain open for seconds to minutes but close in response to elevated calcium levels, low pH, changes in membrane potential, or phosphorylation.
What are the four main molecular mechanisms used by cell-surface receptors for transmembrane signalling?
Cell-surface receptors signal via: (I) ligand-gated ion channels,
(II) receptors with intrinsic guanylyl cyclase activity,
(III) receptors with intrinsic or associated tyrosine kinase activity, and
(IV) G-protein-coupled receptors, which regulate ion channels, adenylyl cyclase, and phosphoinositide-specific phospholipase C.
What are neurotransmitters, and what is their role in signalling?
Neurotransmitters are chemicals released across a neuronal synapse that either promote or inhibit the transmission of a signal between neurons.
What is the primary role of acetylcholine as a neurotransmitter?
Acetylcholine is predominantly an excitatory neurotransmitter, especially at skeletal muscles, but can act as either excitatory or inhibitory at various sites within the CNS and PNS
Where do cholinergic pathways typically begin and end?
Cholinergic pathways generally originate in the basal forebrain complex (BFC) and project to targets in the peripheral nervous system (PNS).
What are the two main types of acetylcholine receptors, and how do they differ?
Acetylcholine acts on muscarinic receptors, which are slow-acting GPCRs, and nicotinic receptors, which are fast ligand-gated ion channels (ionotropic) that open to allow ion flow.
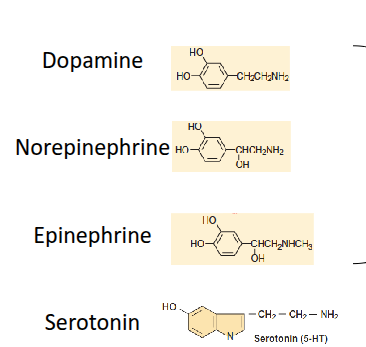
What are biogenic amines, and what structural feature defines the catecholamine subgroup?
Biogenic amines include dopamine, norepinephrine, epinephrine, and serotonin. Catecholamines are characterised by a catechol group with two hydroxyl groups and an ethanolamine side chain containing an amine.
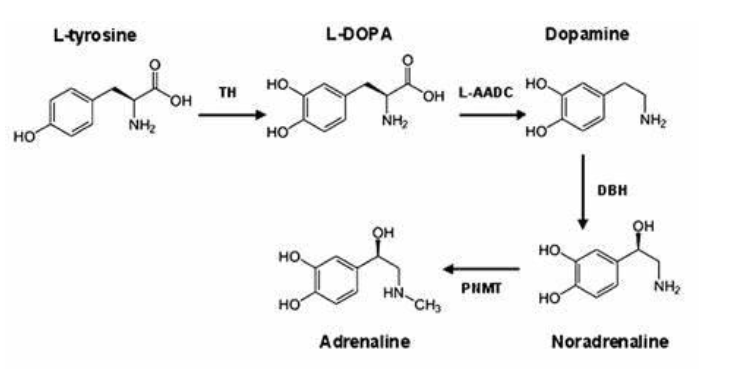
What are the catecholamines, and where are they synthesised?
The catecholamines—dopamine, norepinephrine, and epinephrine—are biogenic amines synthesised by adrenergic neurons. They play key roles in regulating the sympathetic nervous system. They are primarily synthesised in the brain, sympathetic nerve endings, and the adrenal medulla
Biogenic Amines- The Catecholamines
What is dopamine, and where are dopaminergic neurons primarily found?
Dopamine is an excitatory neurotransmitter whose neurons uniquely contain the dopamine transporter (DAT). They are mainly located in the brain, with key dopaminergic circuits projecting from the substantia nigra

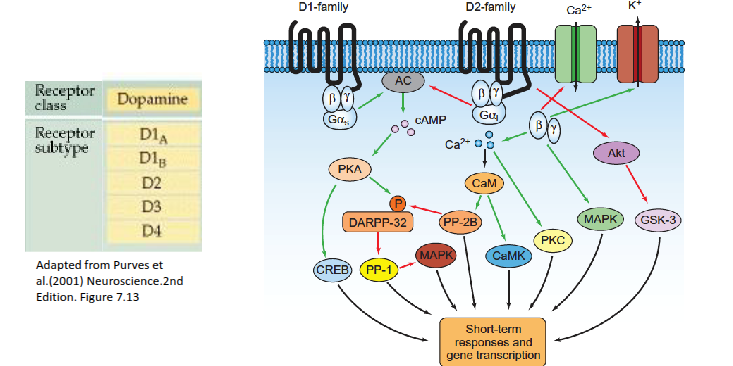
How do dopamine receptors influence intracellular signalling?
Dopamine receptors, mainly found in the brain, alter intracellular signalling by activating or inhibiting adenylyl cyclase, leading to changes in enzyme activity and gene expression.
What are the roles and origins of norepinephrine and epinephrine?
Norepinephrine and epinephrine are generally excitatory neurotransmitters involved in the fight-or-flight response. Norepinephrine-using neurons project from the locus coeruleus, and both neurotransmitters are chiefly synthesised by sympathetic ganglion cells near the spinal cord or abdomen, with epinephrine present at lower levels.
Origins
Norepinephrine is primarily produced by sympathetic neurons throughout the body, where it acts as the main neurotransmitter of the sympathetic nervous system. It is also synthesized in the adrenal medulla, but most of the norepinephrine in the bloodstream comes from nerve endings.
Epinephrine is produced mainly in the chromaffin cells of the adrenal medulla (the inner part of the adrenal glands located on top of the kidneys), where it is converted from norepinephrine by a specific enzyme. It is released into the bloodstream primarily as a hormone.
How many adrenergic receptor types respond to norepinephrine and epinephrine, and can dopamine activate them?
There are nine adrenergic receptor types. At higher doses, dopamine can also activate some adrenergic receptors
What are the main functional differences between α₁, α₂ and β adrenergic receptors?
α₁ receptors cause slow depolarisation by inhibiting K⁺ channels; α₂ receptors cause slow hyperpolarisation by activating different K⁺ channels; β receptors are found in neurons but mainly act on sympathetic muscle targets.
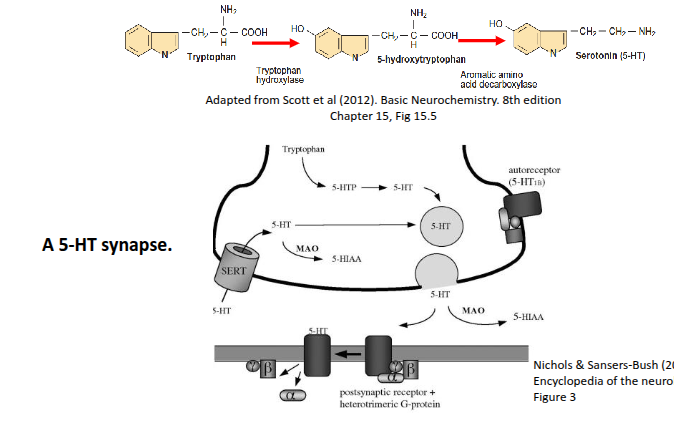
How is serotonin (5-HT) synthesised and how does it function at the synapse?
Serotonin is synthesised from tryptophan in the presynaptic neuron and stored in synaptic vesicles. When an action potential arrives, it is released into the synaptic cleft, where it activates GPCRs on the postsynaptic cell. Excess 5-HT is either degraded by MAO or taken back up by the presynaptic terminal via the serotonin transporter (SERT).
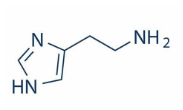
What is histamine, and how does it act in the nervous system?
Histamine is a neurotransmitter that can be either excitatory or inhibitory depending on the receptor it activates. Its receptors are GPCRs, and histaminergic neurons are located mainly in the thalamus and hypothalamus
Histamine Receptors are GPCRs

Amino acids as neurotransmitters
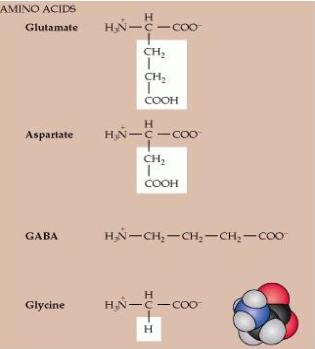
How are glutamate and GABA formed, and in which neurons are they primarily found?
Glutamate derives from α-ketoglutarate in the TCA cycle and is mainly found in glutamatergic neurons. In GABAergic neurons, glutamate can be converted into GABA, providing the major inhibitory neurotransmitter in the CNS.

What is the role of glutamate in neurotransmission, and where does it cycle?
Glutamate is an excitatory neurotransmitter that cycles between neurons and glial cells. It is released at synapses, often alongside zinc.
Where do glutamatergic synapses occur, and how is glutamate stored?
Glutamatergic synapses form between a presynaptic terminal and a postsynaptic dendritic spine or another axon. Glutamate accumulates in synaptic vesicles at 60–250 mM, far higher than its concentration in the cytosol
What are the main types of glutamate receptors and what ions do they conduct?
Glutamate receptors, including those on astrocytes, microglia and oligodendrocytes, include AMPA and kainate receptors that conduct Na⁺, and NMDA receptors that conduct both Na⁺ and Ca²⁺.
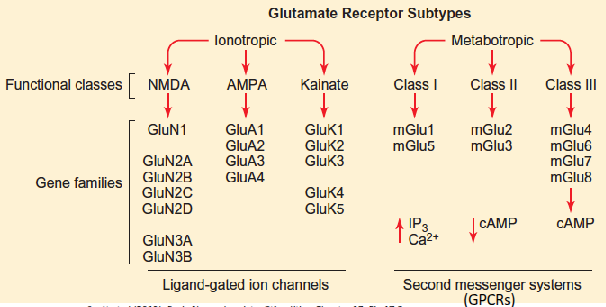
What ions act as allosteric inhibitors of NMDA receptors?
H⁺ (protons) and Mg²⁺ bind to allosteric sites on NMDA receptors and inhibit their activity
What are the main types of ionotropic glutamate receptors and their basic function?
NMDA, AMPA, and kainate receptors are ionotropic glutamate receptors that mediate excitatory synaptic transmission by allowing cation flow, with NMDA receptors also permeable to Ca²⁺.
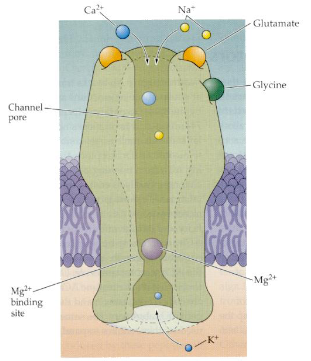
How does glutamate initiate long-term potentiation (LTP) via AMPA and NMDA receptors?
Glutamate binds to AMPA and NMDA receptors, causing Na⁺ influx through AMPA receptors, which depolarises the cell and removes the Mg²⁺ block from NMDA receptors.
What is the role of Ca²⁺ influx through NMDA receptors in LTP?
Ca²⁺ enters the cell through NMDA receptors, activating signalling pathways that increase the number of AMPA receptors in the postsynaptic membrane.
How does LTP affect the postsynaptic neuron's response to future stimuli?
The postsynaptic neuron becomes more sensitive, responding faster and more strongly to glutamate release, enhancing synaptic strength.
What are the key roles of ligand-gated receptors, voltage-gated ion channels, and gap junctions in signal transduction?
Ligand-gated receptors (e.g., acetylcholine) allow communication of nerve impulses between neurons. Voltage-gated Na⁺ and K⁺ channels propagate nerve impulses along the axon. Gap junctions enable rapid, synchronised signalling between cells and permit transfer of small metabolic nutrients.
What are voltage-gated channel receptors, and how do Na⁺ and K⁺ channels differ in structure?
Voltage-gated channels open in response to membrane potential changes. Na⁺ channels consist of four subunits fused into a single polypeptide, whereas K⁺ channels are formed from four separate subunits that assemble together.
How does the ball-and-chain model explain voltage-gated channel inactivation?
A positively charged “ball” attached by a flexible “chain” swings into a negatively charged binding site upon depolarisation, blocking the channel and inactivating it.
What activates ligand-gated channel receptors, and how does the acetylcholine receptor function?
They are activated by extracellular neurotransmitters like glutamate or intracellular second messengers (Ca²⁺, cAMP, cGMP). The acetylcholine receptor is a pentamer, and ligand binding opens the ion pore.
What are gap junctions, and what factors cause them to close?
Gap junctions are intercellular channels allowing direct electrical and metabolic communication. They remain open for seconds to minutes but close in response to elevated Ca²⁺, low pH, membrane potential changes, or phosphorylation.
What are the four main molecular mechanisms used by cell-surface receptors for transmembrane signalling?
(I) Ligand-gated ion channels, (II) receptors with intrinsic guanylyl cyclase activity, (III) receptors with intrinsic or associated tyrosine kinase activity, and (IV) G-protein-coupled receptors (regulating ion channels, adenylyl cyclase, and phospholipase C).
What are neurotransmitters, and what is their role in signalling?
Chemicals released across a neuronal synapse that either promote or inhibit the transmission of a signal between neurons
What is the primary role of acetylcholine as a neurotransmitter?
Predominantly excitatory at skeletal muscles, but can be excitatory or inhibitory at other CNS/PNS sites
Acetylcholine's primary role is as a neurotransmitter for muscle contraction, acting at the neuromuscular junction to excite muscles, and it's crucial for voluntary movement. It also functions extensively in the brain, regulating attention, learning, memory, arousal, and mood, and is a key neurotransmitter in the autonomic nervous system, particularly for parasympathetic functions.
How is GABA synthesised and regulated in GABAergic neurons?
GABA is synthesised from glutamate by glutamic acid decarboxylase, is inhibitory, and high-affinity transporters return it to synaptic terminals for reuse
How do NMDA and AMPA receptors contribute to long-term potentiation (LTP)?
Glutamate binds AMPA/NMDA receptors → Na⁺ influx via AMPA depolarises the cell → Mg²⁺ block removed from NMDA → Ca²⁺ influx activates pathways → more AMPA receptors inserted → postsynaptic neuron becomes more sensitive to future glutamate.
How is GABA action terminated and what is its primary function?
High-affinity transporters return GABA to synaptic terminals for reuse, and GABA acts as an inhibitory neurotransmitter.
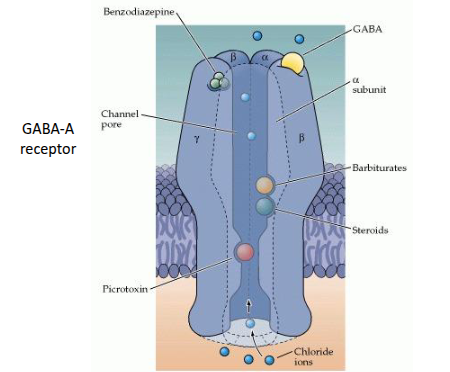
What are the main types of GABA receptors and how do they function?
GABA-A receptors are ligand-gated chloride channels, while GABA-B receptors are G-protein-coupled receptors (GPCRs).
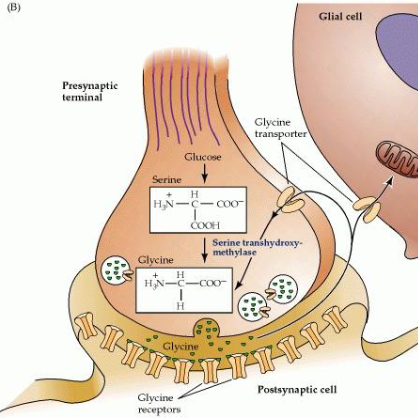
What is glycine, and how does it function as a neurotransmitter?
Glycine is a fast inhibitory neurotransmitter, more localised than GABA, and acts via ligand-gated chloride ion channels
What are neuropeptides, and what are the main groups used as neurotransmitters?
Neuropeptides are signalling molecules that act as neurotransmitters. The main groups are: brain/gut peptides, pituitary peptides, opioid peptides, hypothalamic releasing hormones, and a miscellaneous "catch-all" group.
How do barbiturates affect neurotransmission?
Barbiturates are sedative drugs that enhance GABAergic signalling, increasing inhibitory neurotransmission.
How do benzodiazepines affect neurotransmission?
Benzodiazepines are psychoactive sedatives that promote GABAergic signalling, enhancing inhibitory effects in the CNS
How does alcohol affect neurotransmission?
Alcohol increases GABAergic and dopaminergic signalling, reduces glutamate signalling (especially via NMDA receptors), and decreases serotonin activity
How does cannabis (marijuana) affect neurotransmission?
Cannabis (via 2-AG) modulates signalling of glutamate, GABA, noradrenaline, dopamine, serotonin, and acetylcholine, affecting multiple neurotransmitter systems
Cannabis, primarily through THC, disrupts neurotransmission by binding to CB1 receptors, acting like a "dimmer switch" to reduce the release of neurotransmitters like GABA, glutamate, and dopamine, affecting memory, mood, and reward. It alters the balance of excitatory/inhibitory signals (glutamatergic/GABAergic), impacts serotonin, acetylcholine, and adenosine systems, and can decrease cellular energy (ATP), leading to cognitive deficits, altered mood, and changes in learning processes like LTP.