7: Covalent Bonding
0.0(0)
Card Sorting
1/4
There's no tags or description
Looks like no tags are added yet.
Last updated 10:12 PM on 4/20/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
5 Terms
1
New cards
What is a covalent bond?
The strong electrostatic attraction between a shared pair of electrons and the nuclei of the bonded atoms
2
New cards
What is dative covalent bonding? How do you represent it?
When an atom uses a lone pair of electrons to form a covalent bond
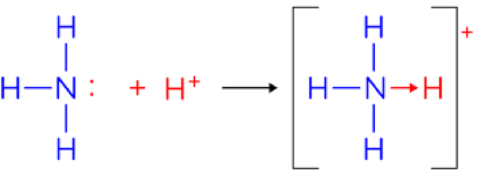
3
New cards
What is a coordinate bond?
A dative bond
4
New cards
What is average bond enthalpy?
A measurement of covalent bond strength
5
New cards
Higher average bond enthalpy means what?
Stronger covalent bond