OIA1012 AROMATIC COMPOUNDS
1/26
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
27 Terms
Definition of Aromatic Compounds
Organic compounds containing benzene or derivatives of benzene. Historically, these compounds often had aromatic odors.
Structure of Benzene
Benzene is a planar hexagonal ring with delocalized π-electrons, represented as a resonance hybrid.
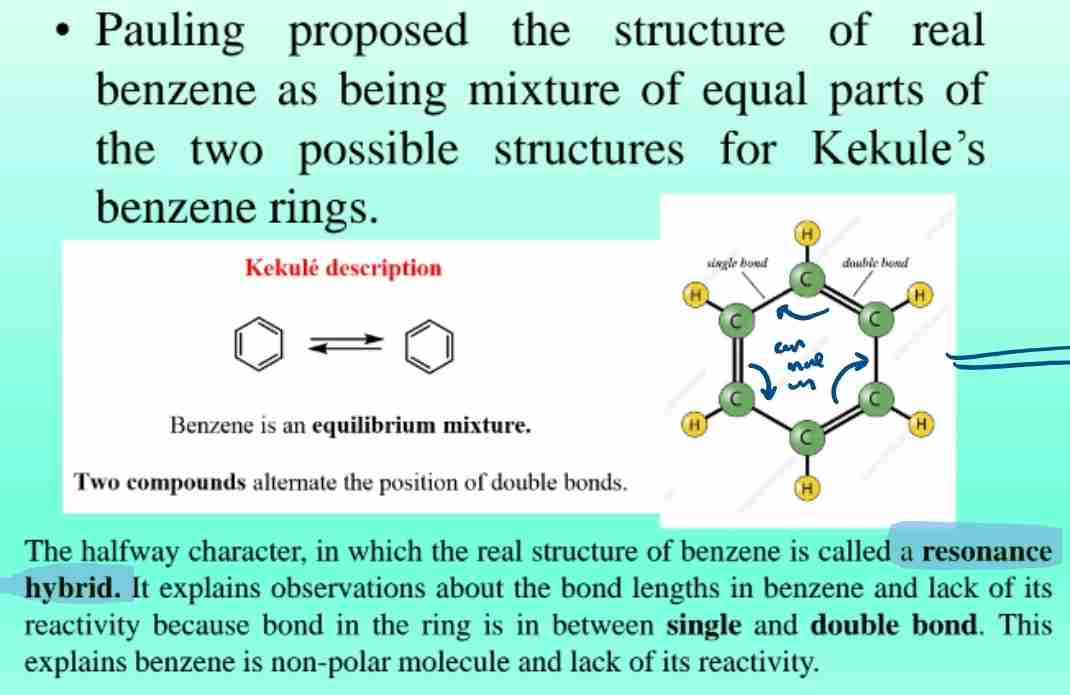
Resonance Hybrid
Proposed by Pauling; explains benzene's stability and uniform bond lengths between single and double bonds.
Aromaticity (Hückel’s Rule)
A compound is aromatic if it has (4n + 2) π-electrons (n = 0, 1, 2, etc.), forming a conjugated planar ring.
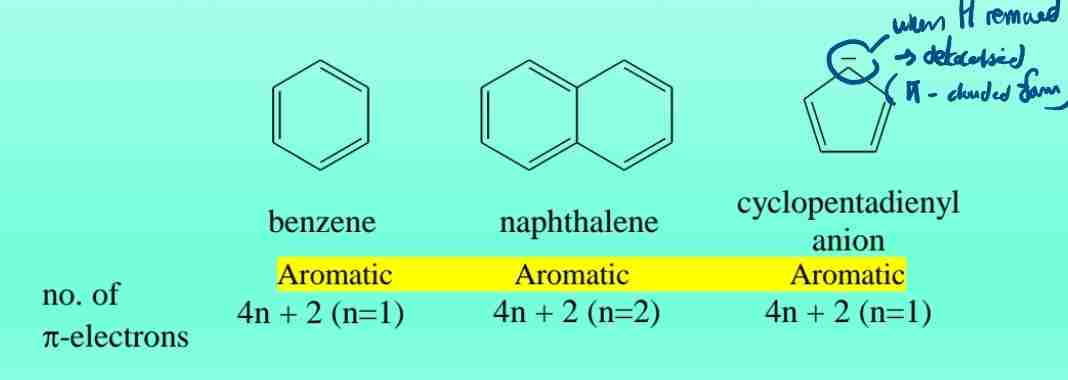
Examples of Aromatic Molecules
- Benzene (6 π-electrons).
- Naphthalene (10 π-electrons).
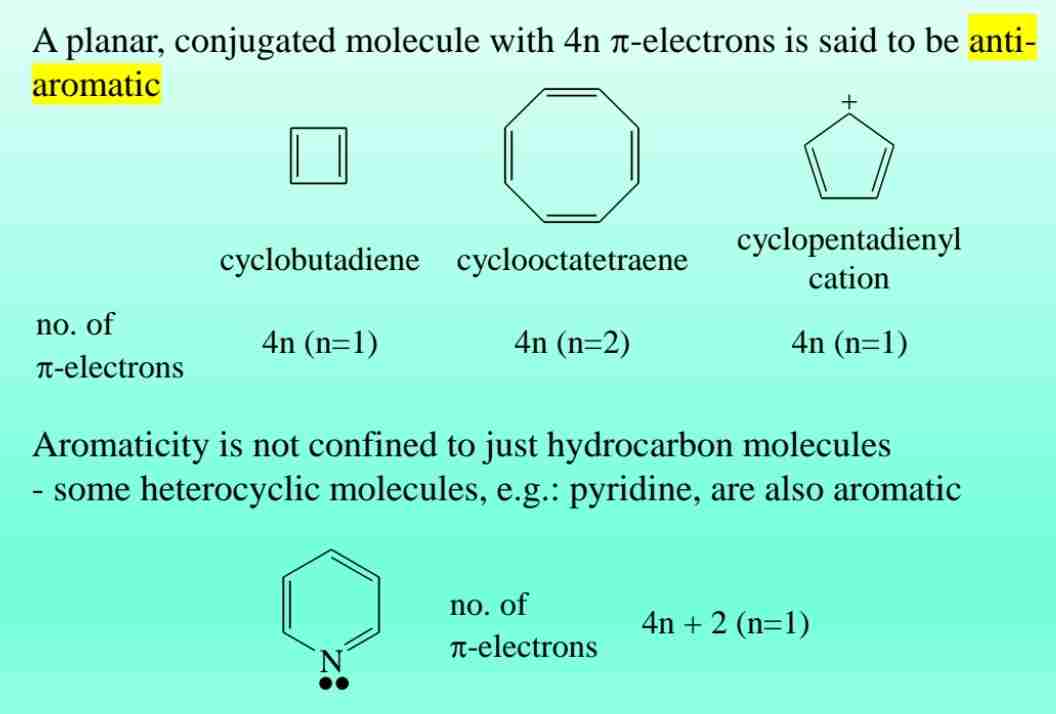
- Pyridine (6 π-electrons, heterocyclic).
Anti-Aromatic Compounds
Planar, conjugated molecules with 4n π-electrons (e.g., cyclobutadiene) are unstable and termed anti-aromatic.
Benzene Synthesis
- Cyclic polymerization of ethyne.
- Reduction of phenols using zinc dust.
Physical Properties of Benzene
- Colorless, flammable liquid.
- Boiling point: 80°C.
- Insoluble in water, miscible with organic solvents.
Aromatic Compound Sources
- Coal tar distillation yields benzene, toluene, and xylene.
- Petroleum catalytic reforming.
Naming Monosubstituted Benzenes
Substituents are named with "-benzene" as the parent (e.g., bromobenzene).
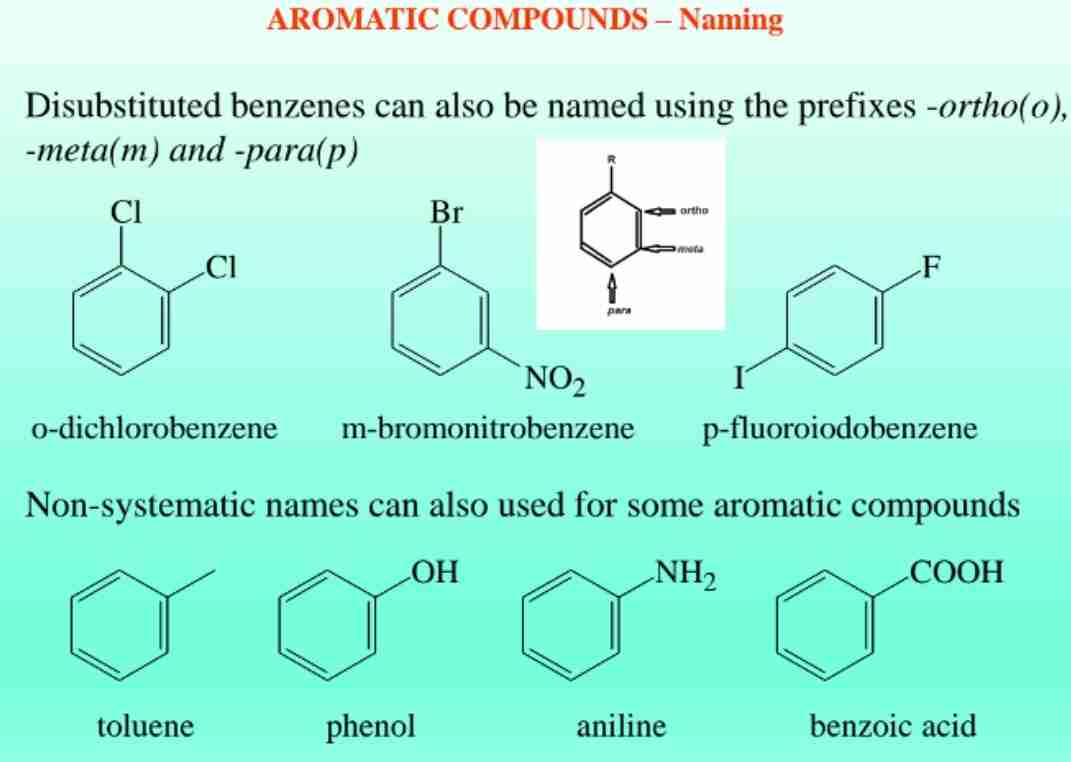
Disubstituted Benzenes
Positions are described as ortho (1,2), meta (1,3), and para (1,4).
Electrophilic Substitution in Benzene
Benzene retains its aromaticity by undergoing substitution reactions rather than addition.
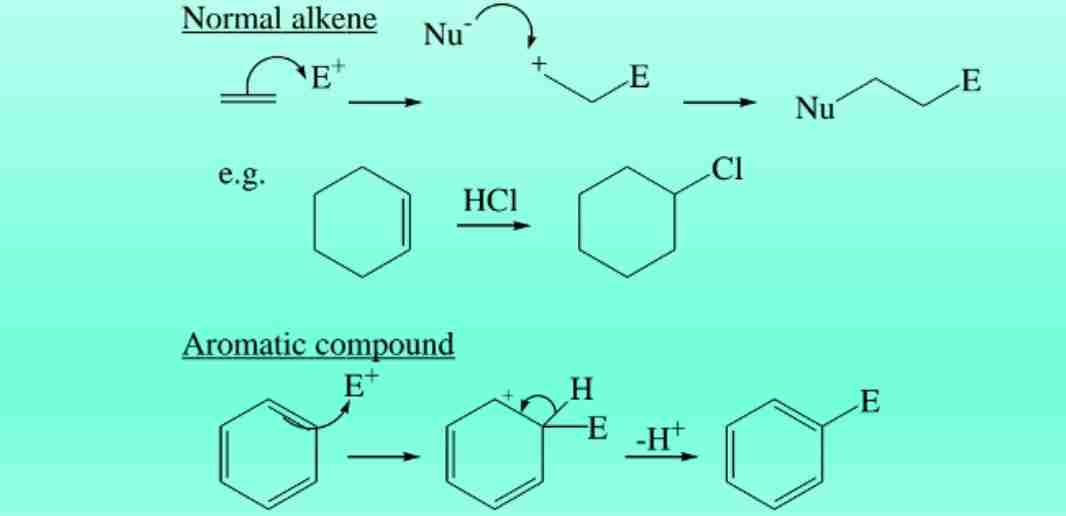
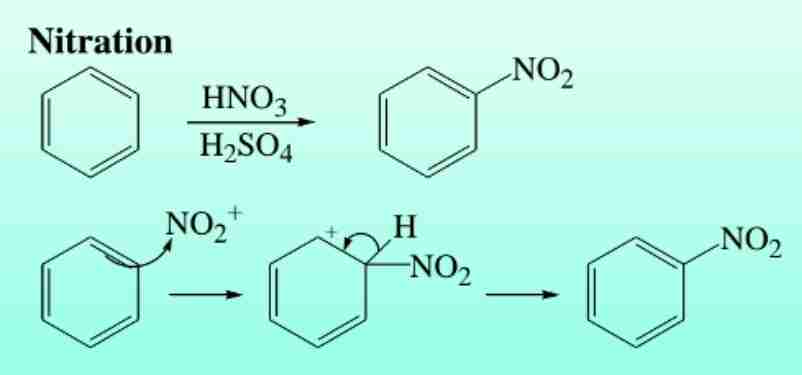
Nitration of Benzene
Reaction with HNO3 in H2SO4 produces nitrobenzene.
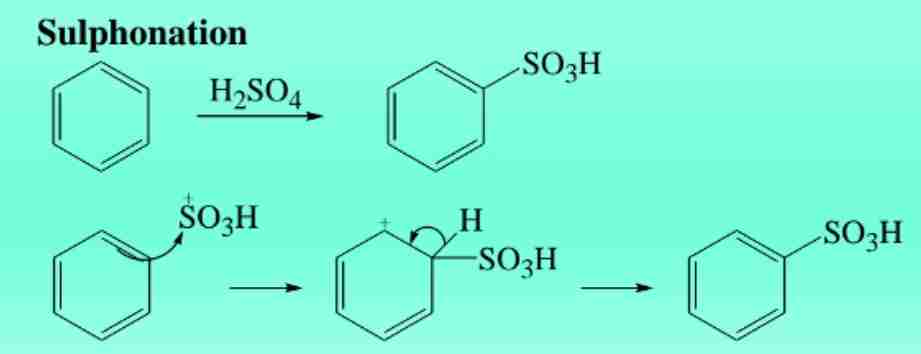
Sulphonation of Benzene
Reaction with H2SO4 yields benzene sulfonic acid.
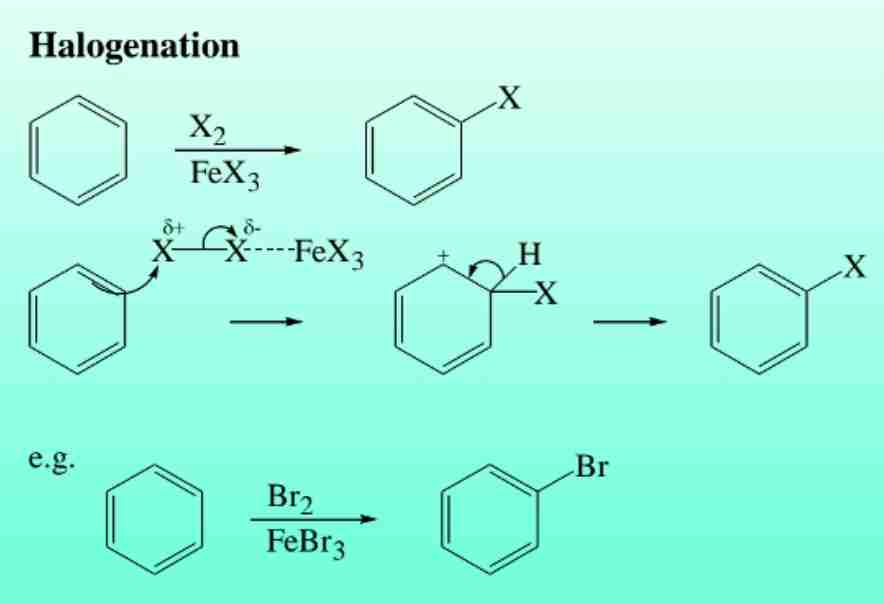
Halogenation of Benzene
Bromination or chlorination in the presence of a Lewis acid catalyst (e.g., FeBr3).
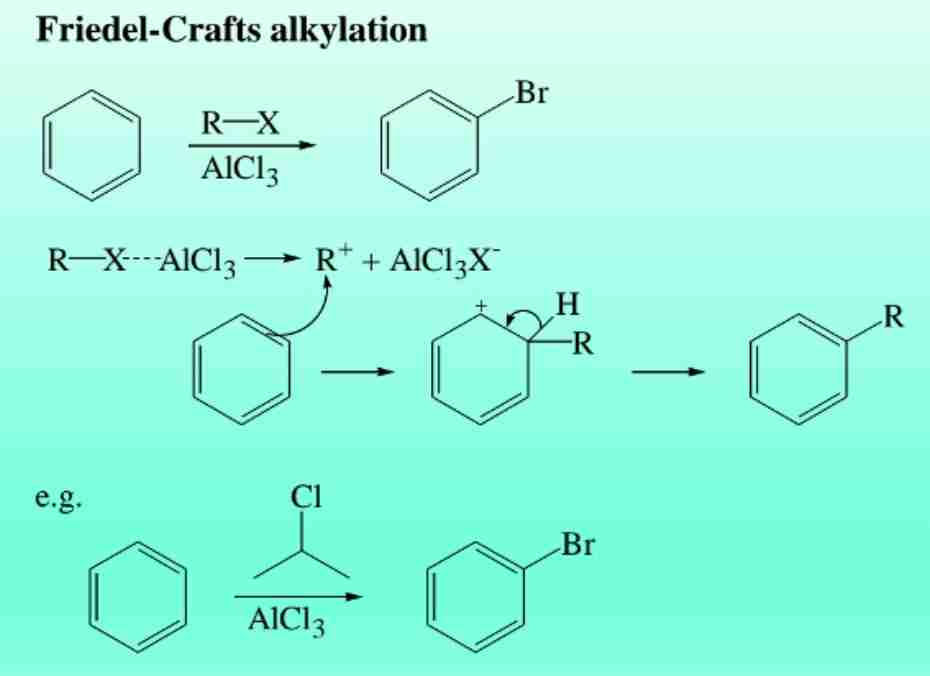
Friedel-Crafts Alkylation
Introduction of alkyl groups using alkyl halides and AlCl3.
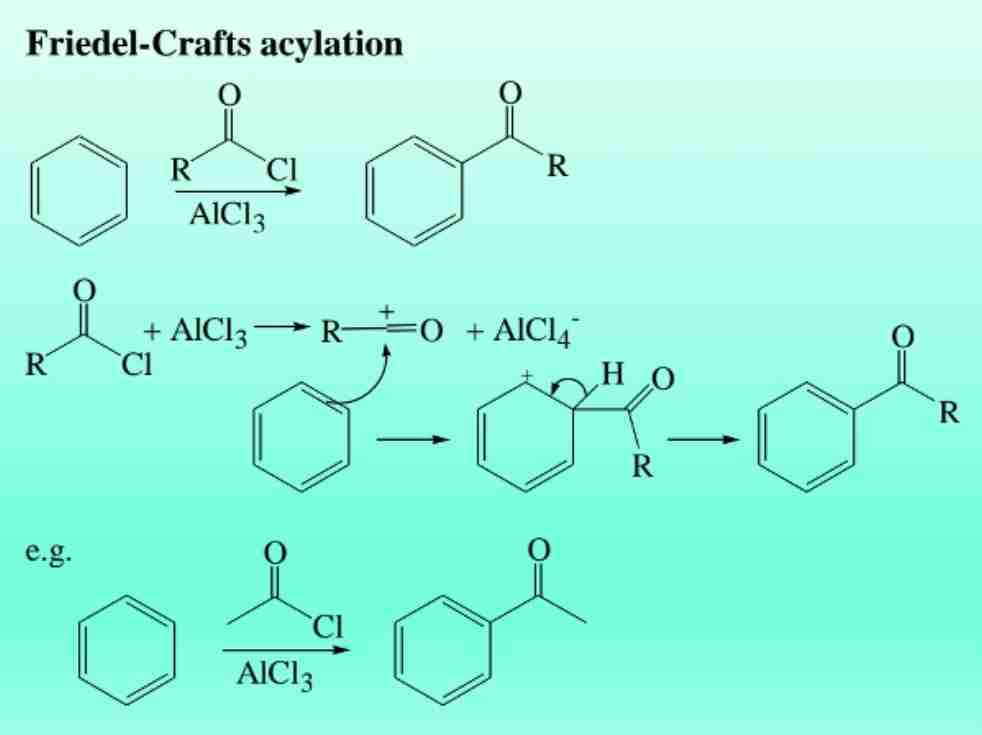
Friedel-Crafts Acylation
Introduction of acyl groups using acyl chlorides and AlCl3.
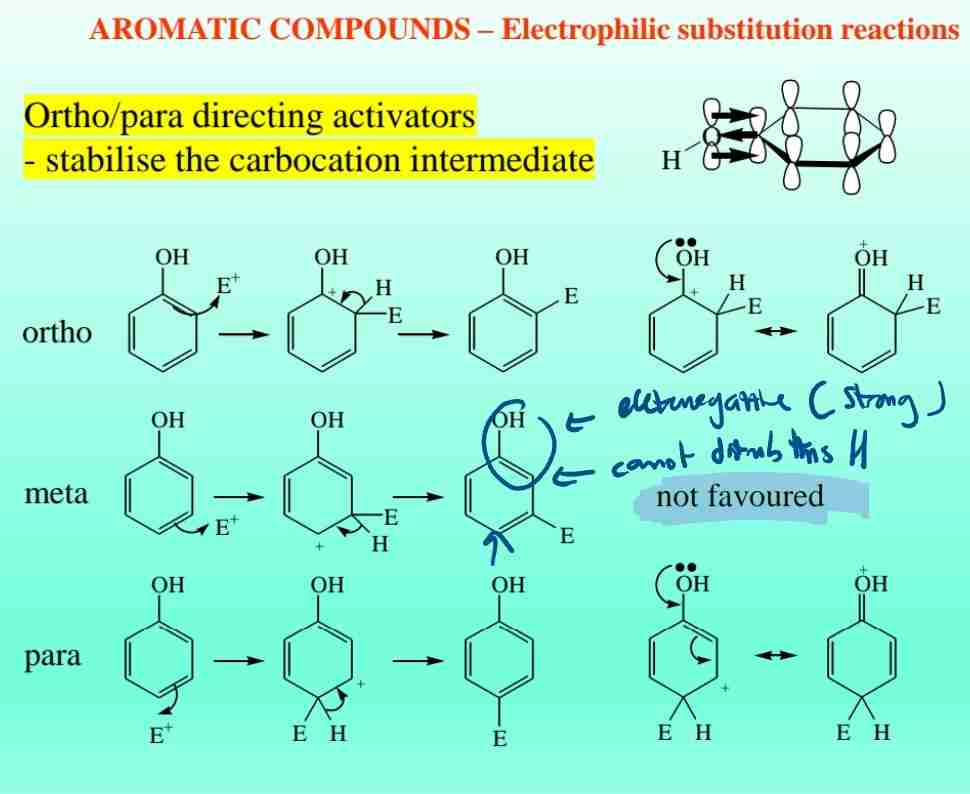
Activating Groups
Electron-donating groups (e.g., OH, NH2) increase reactivity and direct ortho/para substitution.
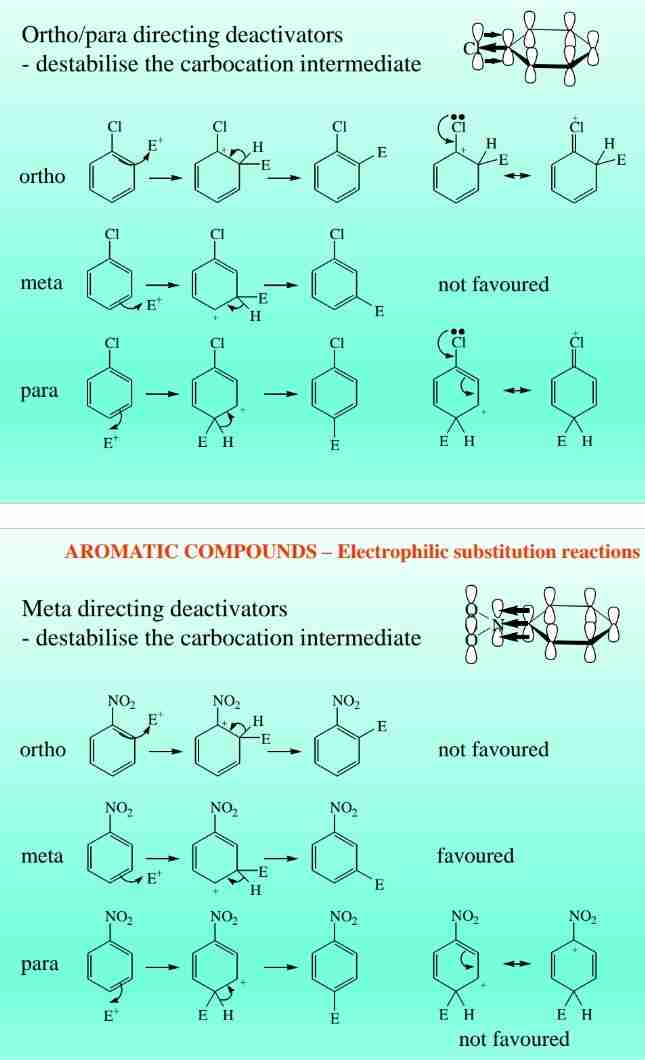
Deactivating Groups
Electron-withdrawing groups (e.g., NO2) decrease reactivity and direct meta substitution.
Addition Effect in Disubstitutued Aromatic Rings
-If directing effects of two groups COMPLEMENT each other: SINGLE PRODUCT formed.
-If directing effects of two groups OPPOSE each other: MORE powerful activating group DOMINANT: MIXTURE PRODUCT formed.
*Due to steric reasons, substitution rarely occurs between 2 group in meta-disubstituted compounds.
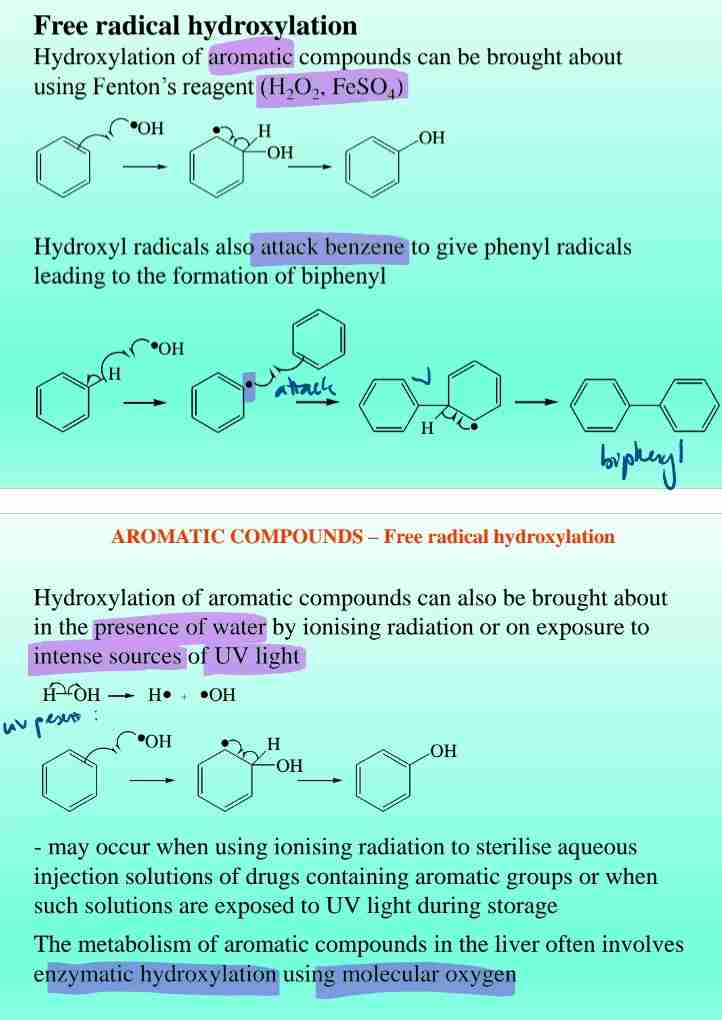
Free Radical Hydroxylation of Benzene
Hydroxylation forms phenols, typically using Fenton’s reagent H2O2 + FeSO4.
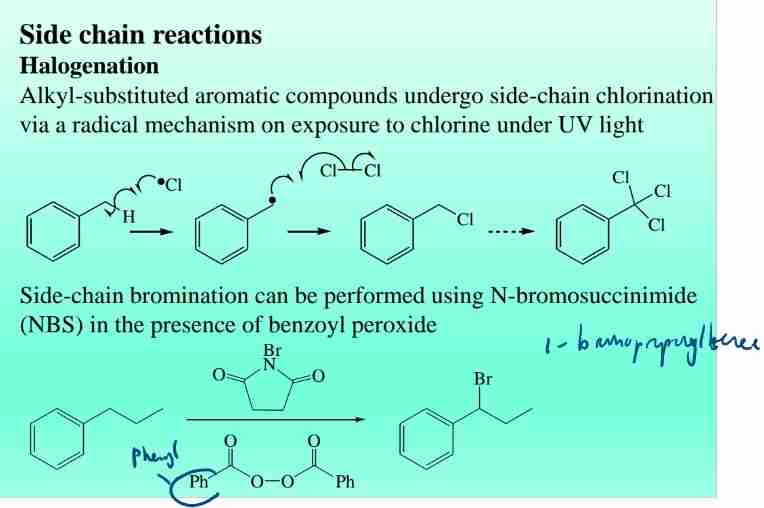
Side Chain Reactions: Halogenation
- Halogenation: alkyl-substituted aromatic compounds undergo Side-Chain chlorination via radical mechanism when expose Cl under UV light
- Bromination: Using N-bromosuccinimide (NBS).
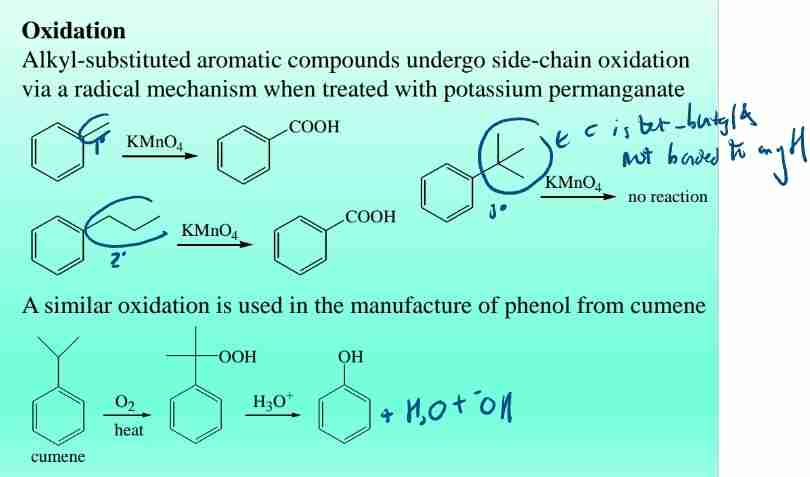
Side Chain Reactions: Oxidation
-Oxidation: Converts alkyl side chains to carboxylic acids using KMnO4.
Biphenyl Formation
Phenyl radicals react to form biphenyl under oxidative conditions.
Carcinogenic Nature of Benzene
Benzene metabolites, such as phenol, are toxic and act as protoplasmic poisons.
Applications of Aromatic Compounds
Found in pharmaceuticals, plastics, and dyes (e.g., aspirin, polystyrene).
Environmental Impacts
Coal tar distillation and petroleum reforming can release harmful aromatic compounds into the environment.