Gibbs Free Energy and Chemical Equilibrium
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
Gibbs Free Energy (ΔG)
Indicates spontaneity of a chemical reaction.
Spontaneous Reaction
Occurs without external energy input; ΔG < 0.
Nonspontaneous Reaction
Requires energy input; ΔG > 0.
Equilibrium Condition
No net change; ΔG = 0.
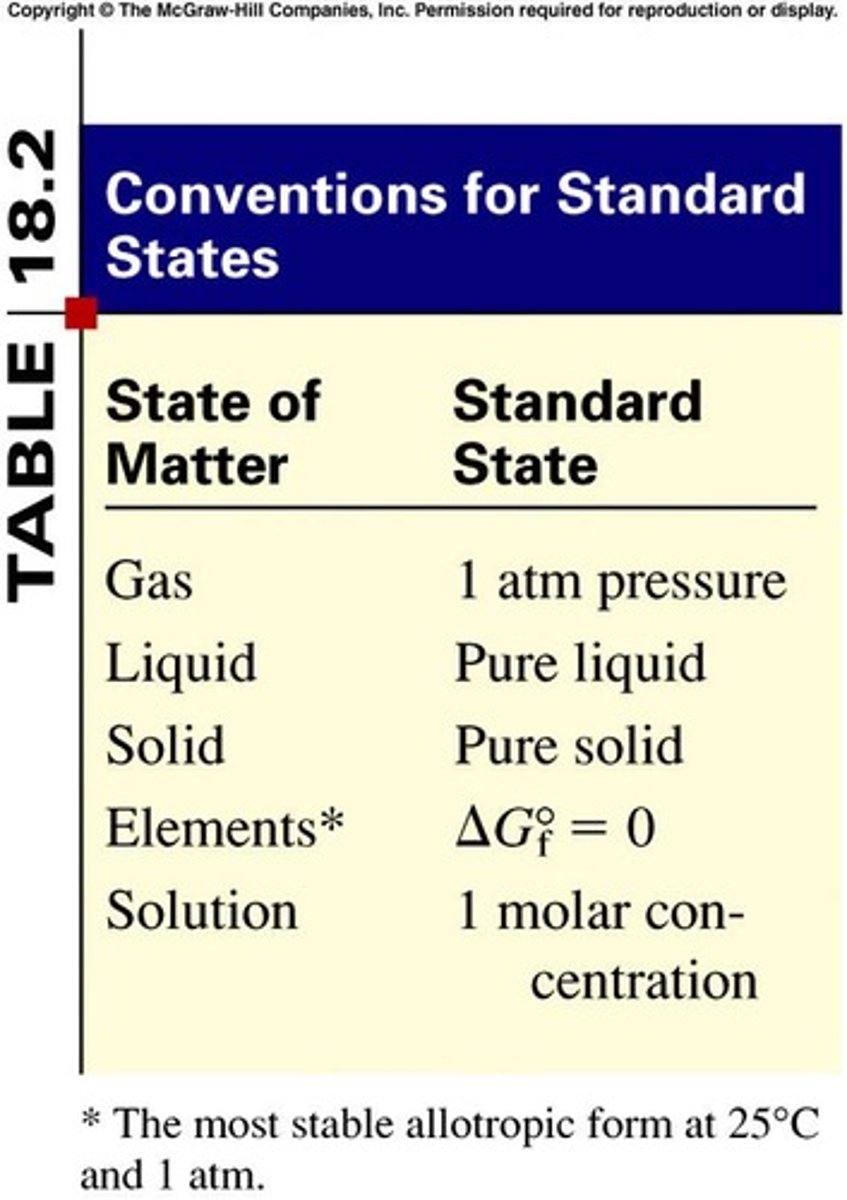
Standard Free Energy Change (ΔG0)
Free energy change under standard conditions.
Reaction Quotient (Q)
Ratio of products to reactants at any time.
Absolute Temperature (T)
Temperature measured in Kelvin (K).
Free Energy Equation
ΔG = ΔHsys - TΔSsys.
Equilibrium Constant (K)
Value of Q at equilibrium; Q = K.
Van't Hoff Equation
Relates equilibrium constants at different temperatures.
Slope of K vs 1/T Plot
Determined by enthalpy change; positive for exothermic.
Enthalpy Change (ΔH0)
Heat change during a reaction at constant pressure.