C9,10,11 Organic Reactions & Polymers (Incomplete!)
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
29 Terms
Alkanes have a __ carbon bond
single
Alkenes have a __ carbon bond
double
alkane general formula
CnH2n+2
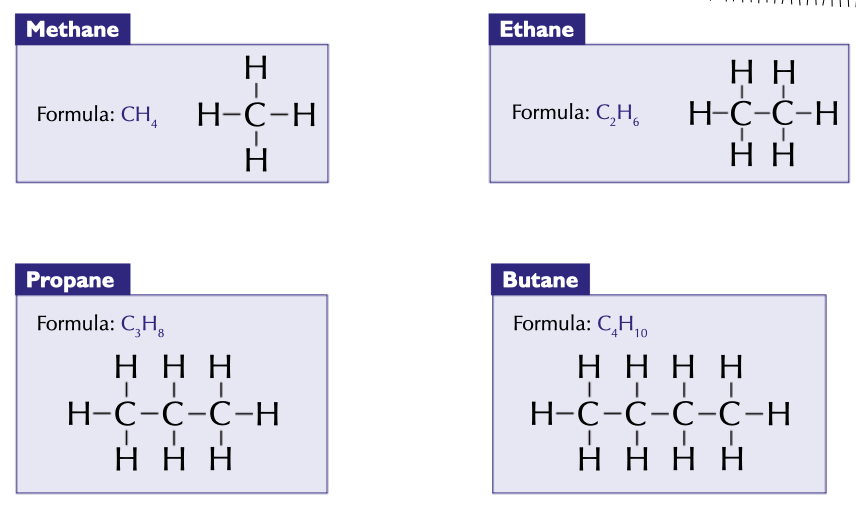
first 4 alkanes, with chemical formulae and displayed formulae
General formula for alkenes
CnH2n
Twice as many hydrogen atoms as carbon atoms.
Alkenes have a ___ bond
C=C double carbon bond
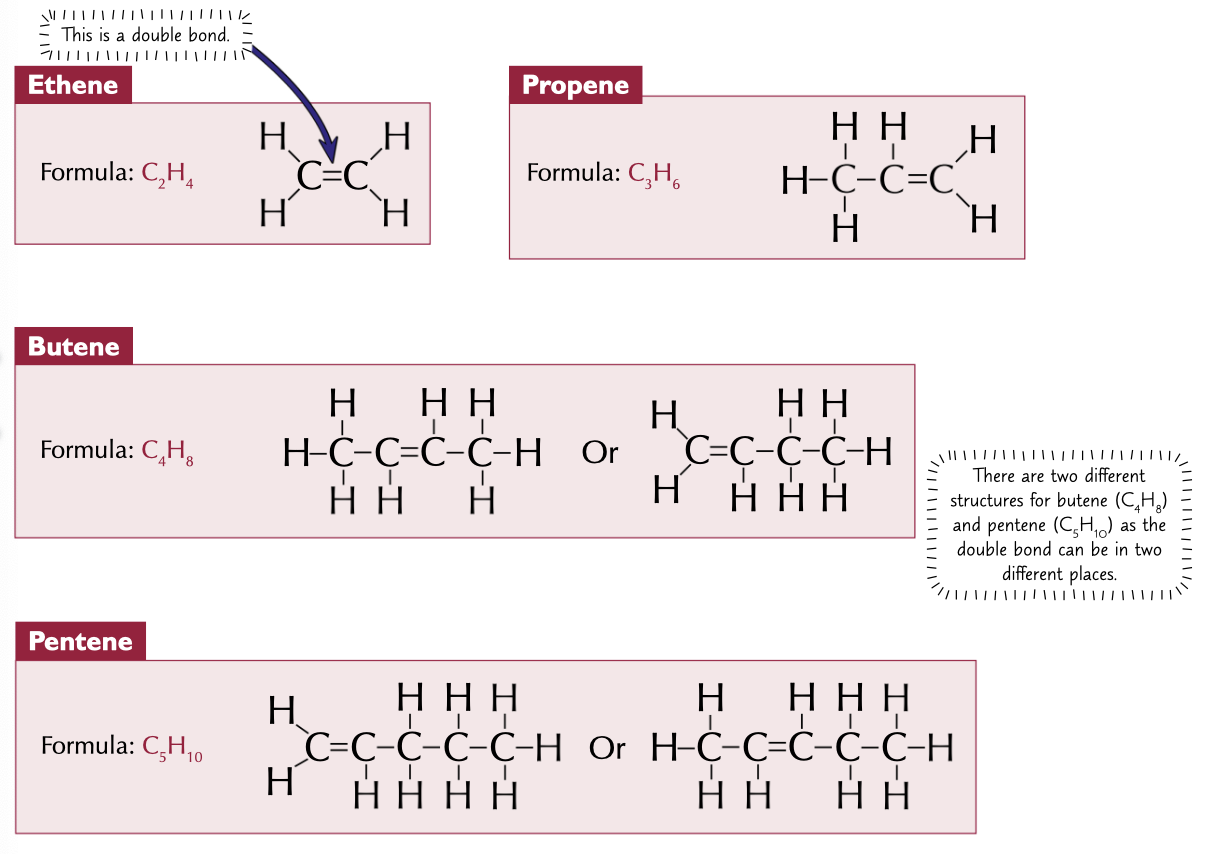
First 4 alkenes, with formulas
Alkanes and alkenes are
Hydrocarbons.
Word equation for complete combustion of any hydrocarbon
Hydrocarbon + Oxygen → Carbon Dioxide + Water
Word equation for incomplete combustion of alkenes
alkene + oxygen → carbon + carbon monoxide + carbon dioxide + water (+energy)
red = extra bit for incomplete. The black bit is the same as complete combustion.
The functional group is
The functional group is a group of atoms in a molecule which determine how that molecule typically reacts
Func. group of alkenes is
C=C
so they all react similarly
Most of the time, alkenes react via ___
alkenes mostly react via Addition reactions
In an addition reaction, what happens?
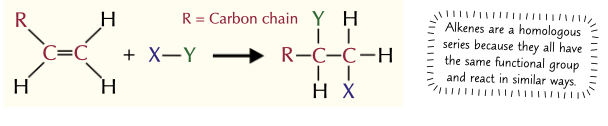
The C=C double bond will open up
to leave a single bond
and a new atom is added to each carbon
Addition of hydrogen to an alkene is known as
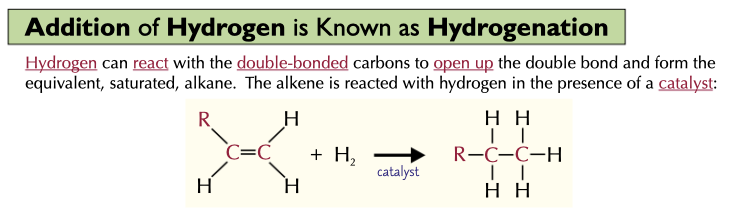
In hydrogenation, what happens?

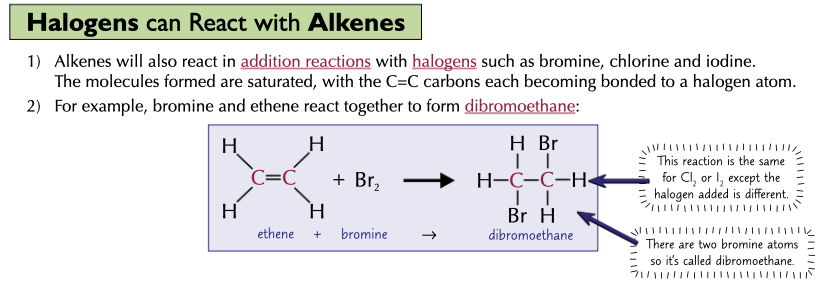
Describe the reaction of a halogen and an alkene
How can you test for alkenes?
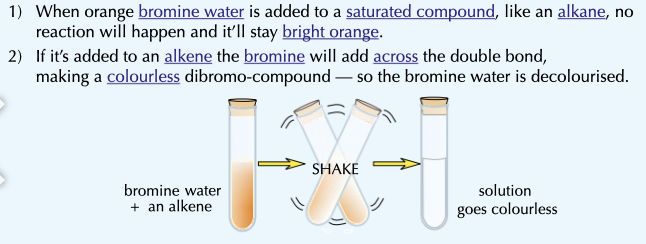
When ORANGE bromine water is added to any saturated compound, like an alkane, no reaction happens.
But when added to an alkene the bromine will add across the double bond, making a COLOURLESS solution. you MUST SHAKE to make it work.
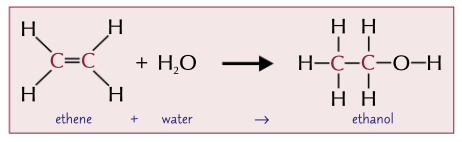
What happens when steam reacts with alkenes
Alcohols are formed.
Water is added across the double bond. There should be a catalyst.
Polymers are long molecules, for4med when lots of small molecules called __ join together.
monomers
monomers → polymer is a reaction called
Polymerisation
Plastics are made up of __
usually __ based
monomers are often __
Plastics are made up of POLYMERS
usually CARBON based
monomers are often ALKENES
The monomers that make up addition polymers have a ___
they are __ monomers
The monomers that make up addition polymers have a double covalent bond
they are unsaturated monomers
When they become polymers, going through addition polymerisation, what happens?