Topic E: Nuclear and Quantum physics
1/86
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
87 Terms
Geiger - Marsden - Rutherford experiment
Experiment which proved that atoms exist mostly of empty space with a dense nucleus in the middle and electron relatively far away.
Atomic Energy Levels
Electrons can only orbit at certain discrete distances from the nucleus. Hence the potential energy of electrons is always in discrete quantities which are classified as energy levels.
Electron transition
When electrons shifts from one energy level to another by absorbing or emitting a photon.
Absorption Spectrum
The range of wavelengths (frequencies) absorbed by an atom.
Emission spectrum
The range of wavelengths (frequencies) emitted by an atom.
Distance to closest approach
The distance of an alpha particle (or any positively charged particle) from the center of a nucleus, where the kinetic energy of the alpha particle is transferred to the electric potential energy of the system due to strong nuclear force acting as soon as the alpha particle approaches that distance.
Wave-particle duality
The concept that light sometimes behaves as a wave and sometimes as a particle. Matter sometimes behaves as a particle and sometimes as a wave.
Photoelectric Effect
Light is shone on the surface of a metal and, if the frequency of the light is greater than the threshold frequency of a metal, photoelectrons are released. It can be demonstrated by using a gold leaf electroscope.
Work function (Φ)
Minimum energy required for a photon to remove an electron from the metal through photoelectric effect.
Stopping Potential Difference/Voltage (Vs)
The minimum voltage across the plate such that the photoelectrons with most initial kinetic energy cannot quite reach the negative plate, which results zero electric current in the photoelectric cell.
Gradient of the relationship Frequency of light vs kinetic energy of the removed electron
Plank's Constant.
X-intercept in the relationship Frequency of light vs kinetic energy of the removed electron
Threshold Frequency of the metal.
Compton Scattering
When x-rays are incident on a carbon target, the scattered wave had a longer wavelength compared to the incident wave. This is further evidence for light as a particle/photon.
Relationship between light intensity and current of the photoelectric cell
Proportional.
Relationship between light intensity and its frequency keeping Luminosity/Power constant
Inverse.
De Broglie wavelength
If light could behave as a particle, then particles must also be able to behave as waves. λ=h/mv.
Isotopes
Different types of atom of an element, with the same number of protons but different numbers of neutrons.
Nuclide
Any specific type of nucleus, where the type is determined by the numbers of protons and neutrons.
Alpha Decay
Emission of alpha particles which compose of helium nucleus (2 protons and 2 neutrons) from the nucleus of the atom.
Beta-minus decay
Emission of beta-minus particles (electrons) from the nucleus. During beta-minus decay the neutron in the nucleus changes to a proton and an electron.
Antineutrino
A particle with zero rest mass and zero charge that results from beta-minus decay.
Positron
A particle with the mass of an electron but a positive charge.
Beta-plus decay
Emission of Beta-plus particles (positrons) from the nucleus. Occurs when proton in the nucleus changes into a neutron and a positron.
Neutrino
A particle with zero rest mass and zero charge that results from Beta-plus decay
Gamma Decay
Emission of gamma rays (high energy photons) from an excited nucleus losing potential energy (mass)
Decay Series
Radioactive decay processes in which the decay of one element creates a new element that may itself be radioactive, this will continue until the sequence ends with stable atoms
Geiger-Müller tube
Detects alpha, beta, gamma radiation when they enter the tube and ionise gas.
Background Radiation
Ionising radiation that is present in the environment
Range of Alpha particle
3-5 centimetres (cm) usually stopped by sheet of paper
Range of Beta particle
25-100 centimetres (cm) usually stopped by 5 mm of aluminium
Range of Gamma ray
Not stopped by air but reduced in intensity in air and significantly reduced by few centimetres of lead/several meters of concrete
Ionisation
Process of removing or adding one or more electrons
Danger of Ionisation
High intensity of ionisation radiation can seriously harm living organisms by damaging or destroying their cells. It can cause serious illness or even death depending on the exposure.
Activity of radioactive nucleus
Number of radioactive decays per unit of time in a sample.
Becquerel (Bq)
A unit of activity where 1 Bq = 1 decay per second
Half-life
Amount of time it takes for half the nuclei in a sample to decay.
Decay constant
The constant of proportionality between the activity of a sample and number of undecided nuclei remaining in the sample.
Strong nuclear force
A force that attracts nucleons in a nucleus. Unlike gravitational force and the electric force, the strong nuclear force has a very short range, only attracting nucleons at separations of about 0.8fm to 3.0fm, 1 fm = 10^-15 m
Nuclear binding energy
Work done to separate a nucleus into its constituent nucleons
Mass defect
Difference between the mass of a nucleus and the total mass of its separated nucleons.
Unified atomic mass unit
Exactly 1/12 the mass of a carbon-12 atom or equal to the mass per nucleon in carbon-12 atom.
Mass-energy equivalence
The concept that mass and energy are the same thing. E=mc^2
Fission
Involves the splitting of an atom's nucleus into two or more smaller nuclei.
Chain reaction
A self-sustaining sequence of nuclear fission reactions in which the products of one reaction trigger further reactions.
Fissile Isotope
Isotopes of elements that are capable of sustaining a nuclear fission chain reaction.
What is fuel enrichment?
The processing of the ore of a nuclear fuel to increase the percentage composition of its fissile isotopes.
Why is fuel enrichment necessary?
If the amount of fissile isotope is too small, the chain reaction won't sustain long enough.
Critical mass
Smallest amount of fissile material needed to sustain a fission chain reaction
Neutron-induced fission
The process of nuclear fission initiated by capturing a slow-moving neutron
Spontaneous fission
Fission process in which a heavy nucleus splits into two equally smaller nuclei without involving a neutron or other particles.
How does nuclear power plant work?
Nuclear and fossil fuel power plants use heat to produce steam, which drives a turbine to generate electricity. When the nuclear reaction happens, the potential energy is converted into kinetic energy of products which then is converted into thermal energy in the nuclear reactor.
Nuclear Reactor
Container consisting of many parts where the nuclear reaction takes place in the power plant.
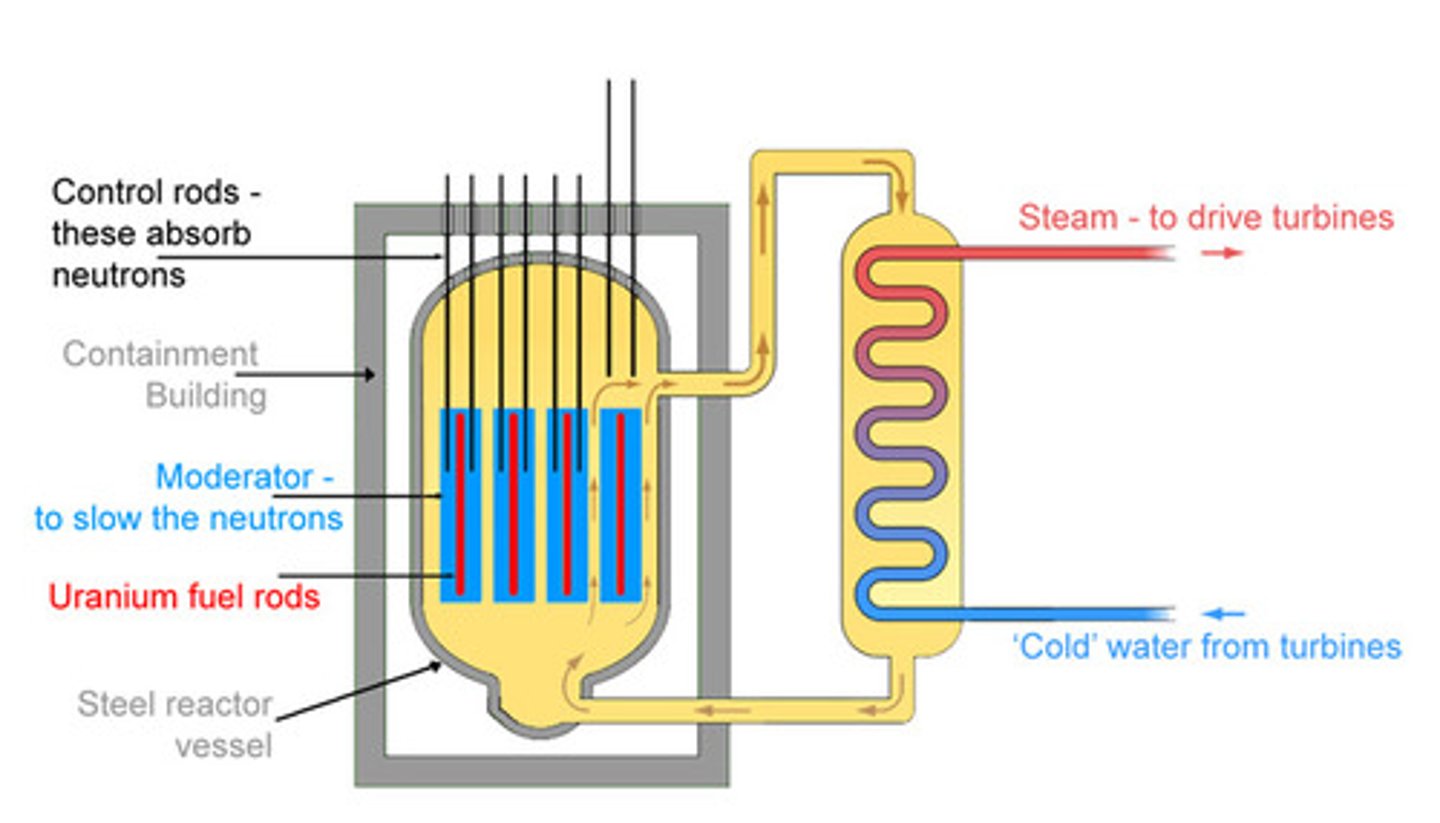
Control rods
Rods made of materials such as boron or cadmium.
Function of Control rods
They absorb some of the neutrons released.
Purpose of control rods
They reduce the rate of chain reaction.
Purpose of shielding in a nuclear reactor
Protects workers and the environment from radiation (especially gamma rays).
Materials used for shielding in a nuclear reactor
A thick layer of concrete and steel.
Shielding in a nuclear reactor
Prevents radiation from escaping into the environment.
Fuel rods
Long cylinders that contain enriched uranium-235.
Moderator
Made of graphite (or water) surrounding the fuel rods.
Purpose of a moderator in a nuclear reactor
To slow down the neutrons released during fission.
Effect of moderator on chain reaction
It makes the neutrons more likely to be absorbed by the uranium 235-nucleus.
Nuclear reactor without a moderator
It would need to use much more enriched uranium to maintain a chain reaction.
Heat exchanger
Transfers heat generated by the nuclear reaction to water, which produces steam that turns the turbines.
Water used in the heat exchanger
Kept separate from the reactor cooling water to prevent radioactive materials from escaping into the environment.
Limitation of nuclear power plant
The products of nuclear fission are highly radioactive isotopes which emit ionising radiation and have long half-lives which can be harmful to human health.
Most common method of storing radioactive waste
Through long-term storage in deep geological repositories.
Plasma
A state of matter made up of freely moving positive ions and electrons.
Creation of plasma
When the gas becomes so hot that its atoms ionise.
Energies of alpha particles and gamma photons
They always have discrete energies.
Observation of discrete energies in radioactive decay
Leads to a model in which the energy of a nucleus is quantised.
Energy states of a nuclide
Each nuclide has a lowest energy state (ground state) and a particular set of possible excited states.
Nucleus after alpha decay in an excited state
It will lose energy by emitting one or more gamma photons until it reaches its ground state.
Emitting an alpha particle leaves the nucleus in its ground state
There will be no subsequent emission of gamma photons.
Most alpha sources
Also sources of gamma rays because alpha decay often leaves the nucleus in an excited state, which then emits gamma photons.
Spectrum of energies of beta particles
A continuous spectrum of energies.
Particle emitted during beta decay
Antineutrino in beta-plus decay and neutrino in beta-minus decay.
Nuclear Fusion
The process of atomic nuclei joining together to form a larger nucleus releasing large amounts of energy.
Proton-proton (P-P) chain
The series of nuclear reactions that begins with four protons and ends with a helium-4 nucleus.
Conditions for fusion
The nuclei need to have an incredibly high velocity, enough that their kinetic energy can supply the work needed to overcome electrostatic repulsion and must be very close to one another for the strong nuclear force to act.
Luminosity
The amount of energy a black body emits per second in the form of electromagnetic radiation.
Stars on the main sequence
Stars that mainly fuse hydrogen in their core.
Temperature characteristic of main sequence stars
They range from cool red dwarfs to hot hyper-giants.
Lifespan of red dwarfs
Up to a trillion years.
Lifespan of hyper-giant stars
Only a few million years.
Phase stars spend the majority of their lives in
The main sequence phase.
Instability strip
Stars in this region tend to ‘pulsate’, varying in size and luminosity. Most stars that are more massive than the Sun spend some of their lifetime in this