Organic Chemistry
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
How is crude oil formed
it is formed over a billion of years due to the effects of high pressures and temperatures on ancient organic matter such as plankton
What is a hydrocarbon
A compound that only contains hydrogen and carbon
What is the general formula of the homologous series of alkanes?
Cn H2n+2
What is molecular formula
the actual number of each atom in a molecule, one molecule at a time
such as C4H10
What is displayed formula
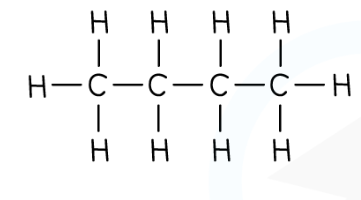
What is structural formula

What are the first four alkanes?
Methane, ethane, propane, butane
How does fractional distillation work?
Heating crude oil and waiting for it to vaporise
the vapours of the crude oil enter the fractionating column which has a temperature gradient
The vapours of hydrocarbons with high boiling points condense at the bottom of the column
The vapours of hydrocarbons with lower boiling points rise up the column and condense at the top
what is kerosene used for (near the middle of fractionating column)?
Jet fuel for aircrafts
what is LPG ( liquid petroleum gas) used for
Domestic heating and cooking
What is petrol used for
Car fuel
What is diesel used for?
Diesel engines
What is heavy fuel oil used for
Ships and power stations
What useful materials are made out of hydrocarbons?
solvengts, lubricants, polymers and detergents
What is the trend in boiling points for hydrocarbons?
The bigger the hydrocarbon, the higher it's boiling point
This is because the size of the intermolecular forces increases requiring more energy to overcome them
What is the trend in viscosity for hydrocarbons and why does it determine how useful a hydrocarbon is?
Viscosity also increases with increasing chain length
This is also due to the increased intermolecular forces of attraction as molecular size increases
Longer chain hydrocarbons would not be as useful as fuels for cars as they would be too thick and clog the engine.
Thus they are more useful as lubricants as they are less likely to burn and function to reduce friction between moving parts
How does molecular size also affect flammability and the uses of hydrocarbons
Smaller hydrocarbon molecules are more flammable and are easier to ignite than larger molecules
This makes them very useful as fuels, releasing large amounts of energy when they burn
what are the products of complete combustion of hydrocarbons?
carbon dioxide and water, the carbon and hydrogen are oxidised.
When balancing a combustionequation, what order do you balance the elements
1) Carbon
2) Hydrogen
3) Oxygen
What should the temperature of a furnace used to heat a fractionating column be?
between 400 - 500
What are the condiitons needed for steam cracking?