Chem 100 Lec: EXAM 1 PRACTICE
1/136
Earn XP
Description and Tags
CHEM 100 LEC
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
137 Terms
Aluminum
AI
Antimony
Sb
Barium
Ba
Bromine
Br
Calcium
Ca
Carbon
C
Cesium
Cs
Chlorine
Cl
Cobalt
Co
Copper
Cu
Fluorine
F
Gold
Au
Hydrogen
H
Iodine
I
Iron
Fe
Lead
Pb
Lithium
Li
Magnesium
Mg
Mercury
Hg
Nickel
Ni
Nitrogen
N
Oxygen
O
Phosphorus
P
Potassium
K
Rubidium
Rb
Silicon
Si
Silver
Ag
Sodium
Na
Strontium
Sr
Sulfur
S
Tin
Sn
Zinc
Zn
AI
Aluminum
Sb
Antimony
Ba
Barium
Br
Bromine
Ca
Calcium
C
Carbon
Cs
Cesium
Cl
Chlorine
Co
Cobalt
Cu
Copper
F
Fluorine
Au
Gold
H
Hydrogen
I
Iodine
Fe
Iron
Pb
Lead
Li
Lithium
Mg
Magnesium
Hg
Mercury
Ni
Nickel
N
Nitrogen
O
Oxygen
P
Phosphorus
K
Potassium
Rb
Rubidium
Si
Silicon
Ag
Silver
Na
Sodium
Sr
Strontium
S
Sulfur
Sn
Tin
Zn
Zinc
What is the Smallest Unit to Largest Unit according to their Magnitude
Microliter
Milliliter
Centiliter
Deciliter
Liter
Kiloliter
What is the Definition of Specific Heat?
The amount of heat required to increase the temp of 1g of a substance by 1 °C
What are the Names for Diatomic Elements?
Bromine
Hydrogen
Iodine
Fluorine
Nitrogen
Oxygen
Chloride
What are the Chemical Formulas for Diatomic Elements?
Br2
H2
I2
F2
N2
O2
Cl2
Would the properties change when a solid like dry ice is transformed to gas?
The Mass is
Same
Would the properties change when a solid like dry ice is transformed to gas?
The Composition is
Same
Would the properties change when a solid like dry ice is transformed to gas?
The Density is
Changes/Different
Would the properties change when a solid like dry ice is transformed to gas?
The Physical State is
Changes/Different
Is N2 an Element or a Compound?
Element
Is CO2 an Element or a Compound?
Compound
Is O2 an Element or a Compound?
Element
Is He an Element or a Compound?
Element
Is NO2 an Element or a Compound?
Compound
When you have Dissolved a Small Amount of Sugar in Water, you have prepared What?
A Mixture
Is Sugar Dissolved in Water Homogeneous or Heterogeneous?
Homogeneous
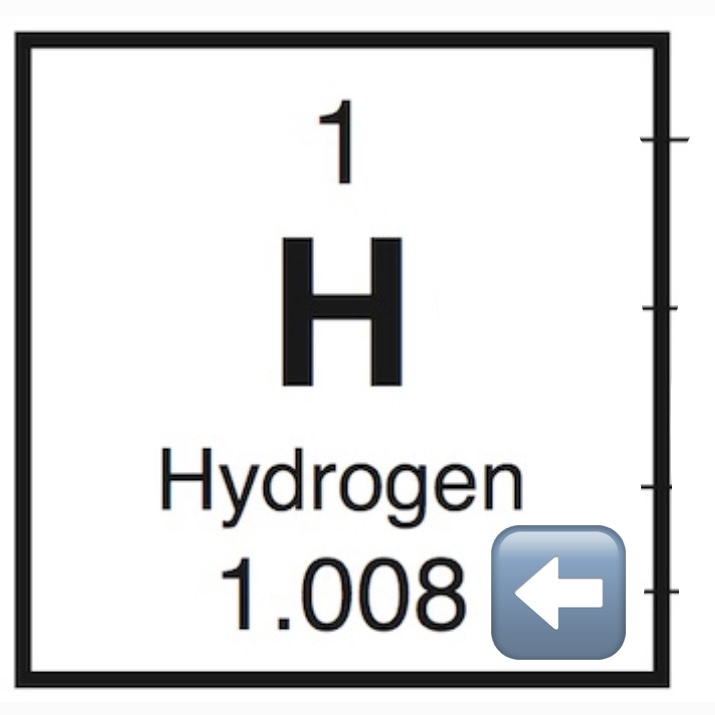
Atomic Mass
The Kinetic Energy of the Particles Increases as the Temperature blanks
Increases
True or False: The pressure exerted on the inner wall of a vessel containing a sample of gas is due to the collisions of the gas particles with the inner wall
True
True or False: H3PO4 is a molecule of a compound
True
True or False: H3PO4 is a homogeneous mixture
False
True or False: H3PO4 is a heterogeneous mixture
False
True or False: H3PO4 is an element
False
True or False: H3PO4 is a compound
True
True or False: A compound is a pure substance made of elements which are combined chemically
True
True or False: A compound is a pure substance that can be decomposed into components (That is, into simpler substances) by chemical means only
True
True or False: A compound is a pure substance that can be decomposed into components (That is, into simpler substances) by physical means only
False
When Heat is Added to a Mixture of Ice and Water at 0 °C, we expect the Temperature of the Mixture to:/ Explain your Answer
Remain the same. Heat is used to break the bonds (Energy) between the particles in ice. The temperature will increase only after all the ice has melted.
True or False: Compounds cannot be decomposed into simpler substances
False
True or False: Compounds can be decomposed by physical means into simpler substances
False
True or False: Compounds can be decomposed into simpler substances by chemical means
True
True or False: Mixtures cannot be decomposed into simpler substances
True or False: Mixtures can be decomposed by physical means into simpler substances
Energy due to Position or Composition is Called
Potential Energy
Energy due to Motion is Called
Kinetic Energy
Give the Names of Any Transition Elements (Name Three)
Scandium
Titanium
Vanadium
Chromium
Manganese
Iron
Cobalt
Nickel
Copper
Zinc
Yttrium
Zirconium
Niobium
Molybdenum
Technetium
Ruthenium
Rhodium
Palladium
Silver
Cadmium
Hafnium
Tantalum
Tungsten
Rhenium
Osmium
Iridium
Platinum
Gold
Mercury
Give the Chemical Symbols of any Transition Elements (Name Three)
Sc
Ti
V
Cr
Mn
Fe
Co
Ni
Cu
Zn
Y
Zr
Nb
Mo
Tc
Ru
Rh
Pd
Ag
Cd
Hf
Ta
W
Re
Os
Ir
Pt
Au
Hg
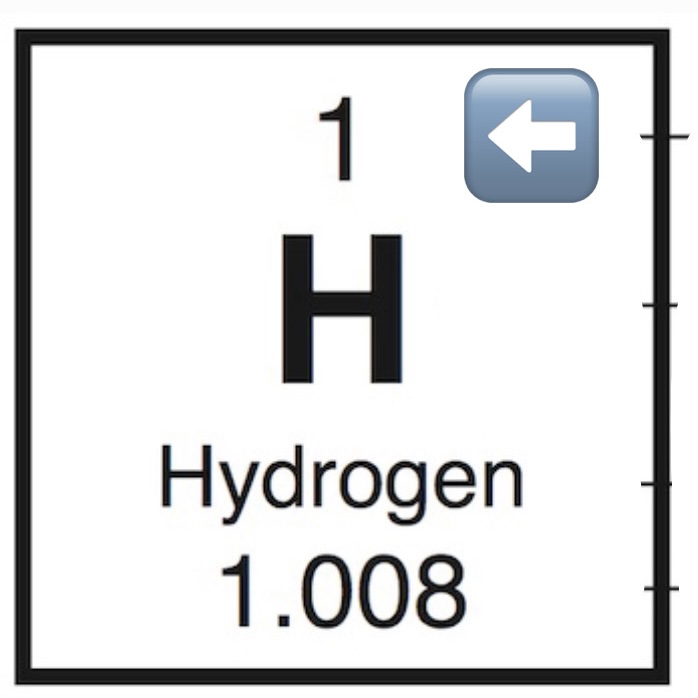
Atomic Number