Molecular Geometry and Bond Angles
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
Linear bond angle
180o
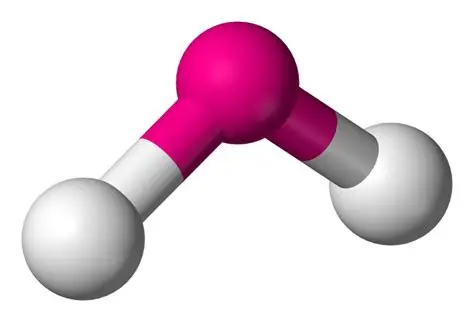
bent bond angle
104.5o
trigonal planar bond angle
120o
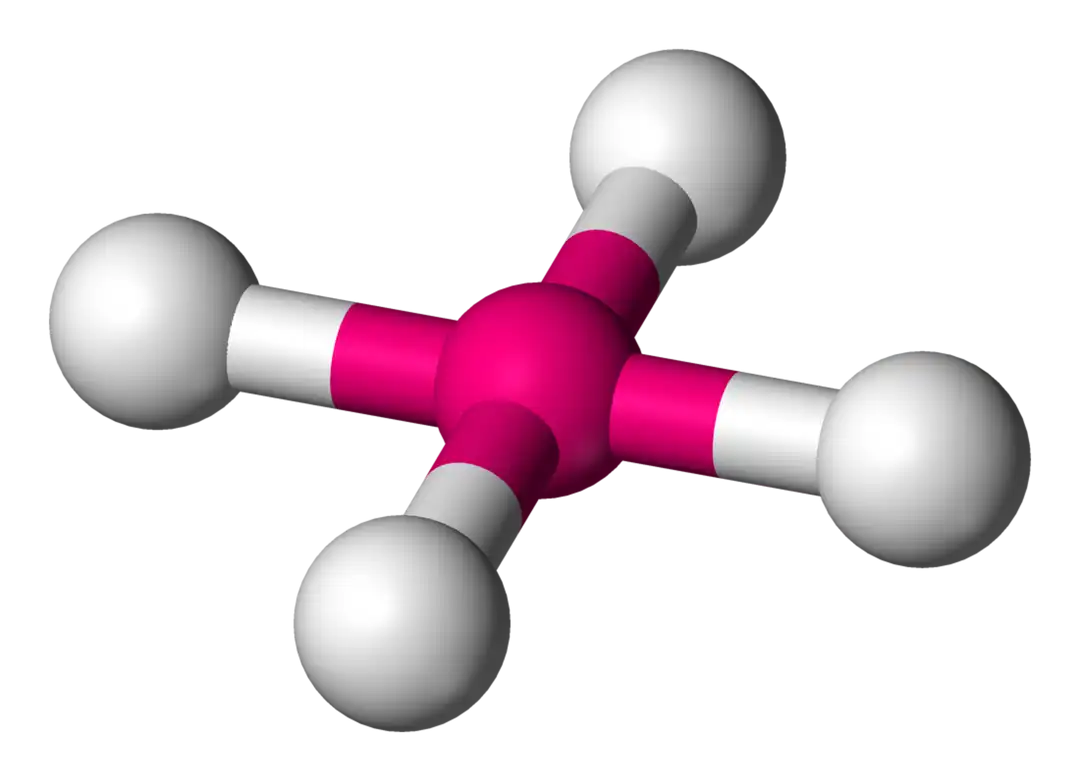
tetrahedral bond angle
109.5o
trigonal pyramidal bond angle
107.5o
trigonal bipyramid bond angle
90o, 120o
octahedral bond angle
90o
seesaw bond angle
90o, 120o
t shaped bond angle
90o, 180o
square planar bond angle
90o
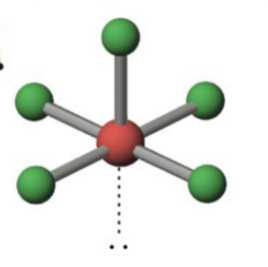
Trigonal bipyramidal
5 electron domains, 0 lone pairs
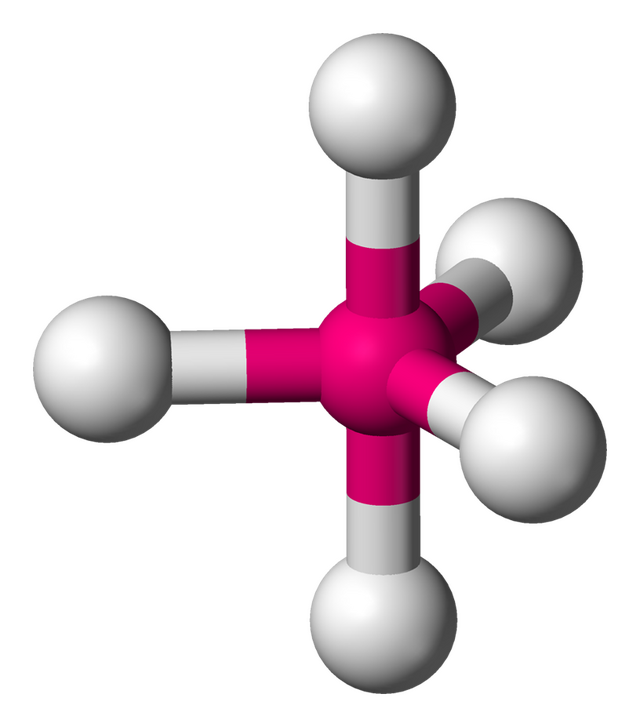
tetrahedral
4 electron domains, 0 lone pairs
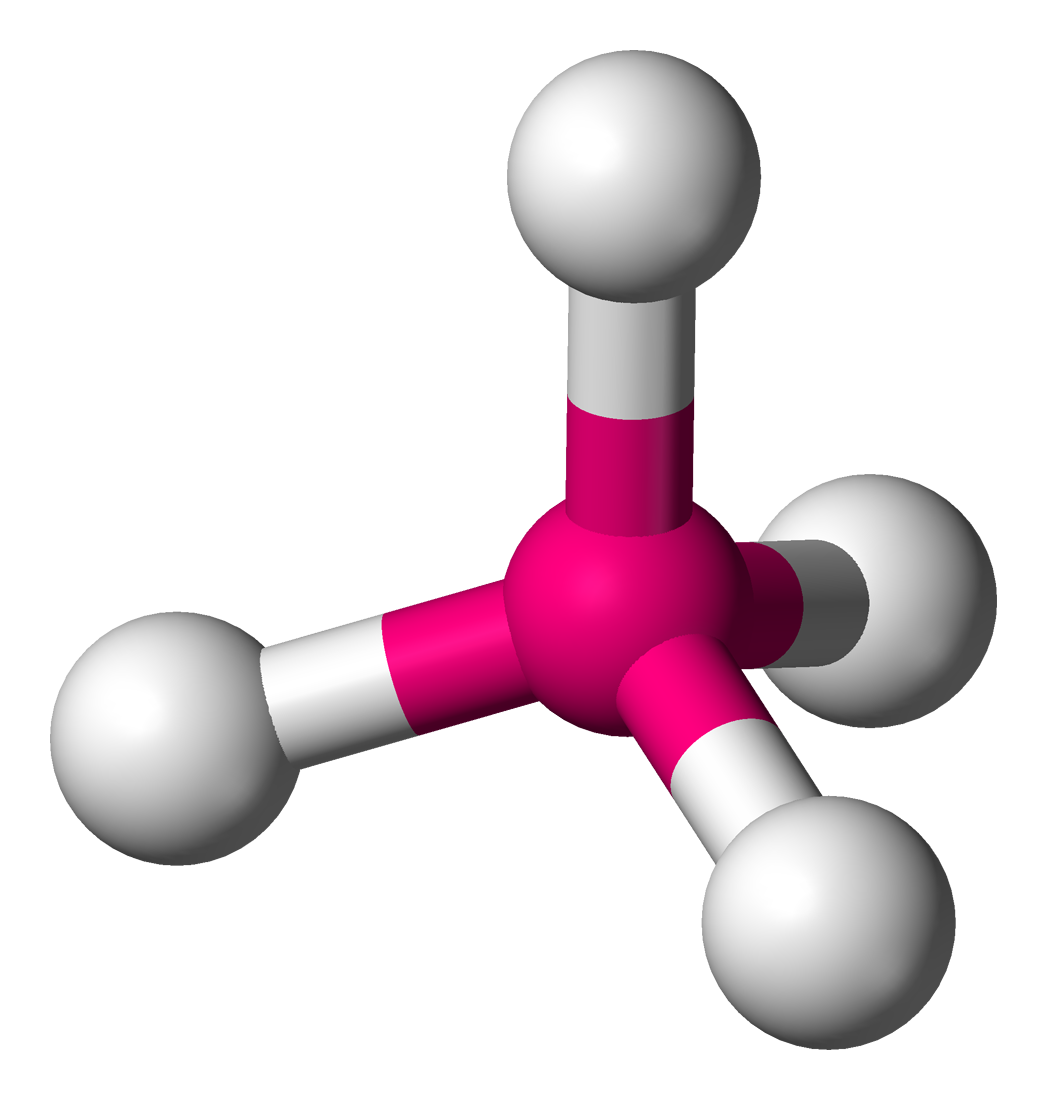
octahedral
6 electron domains, 0 lone pairs
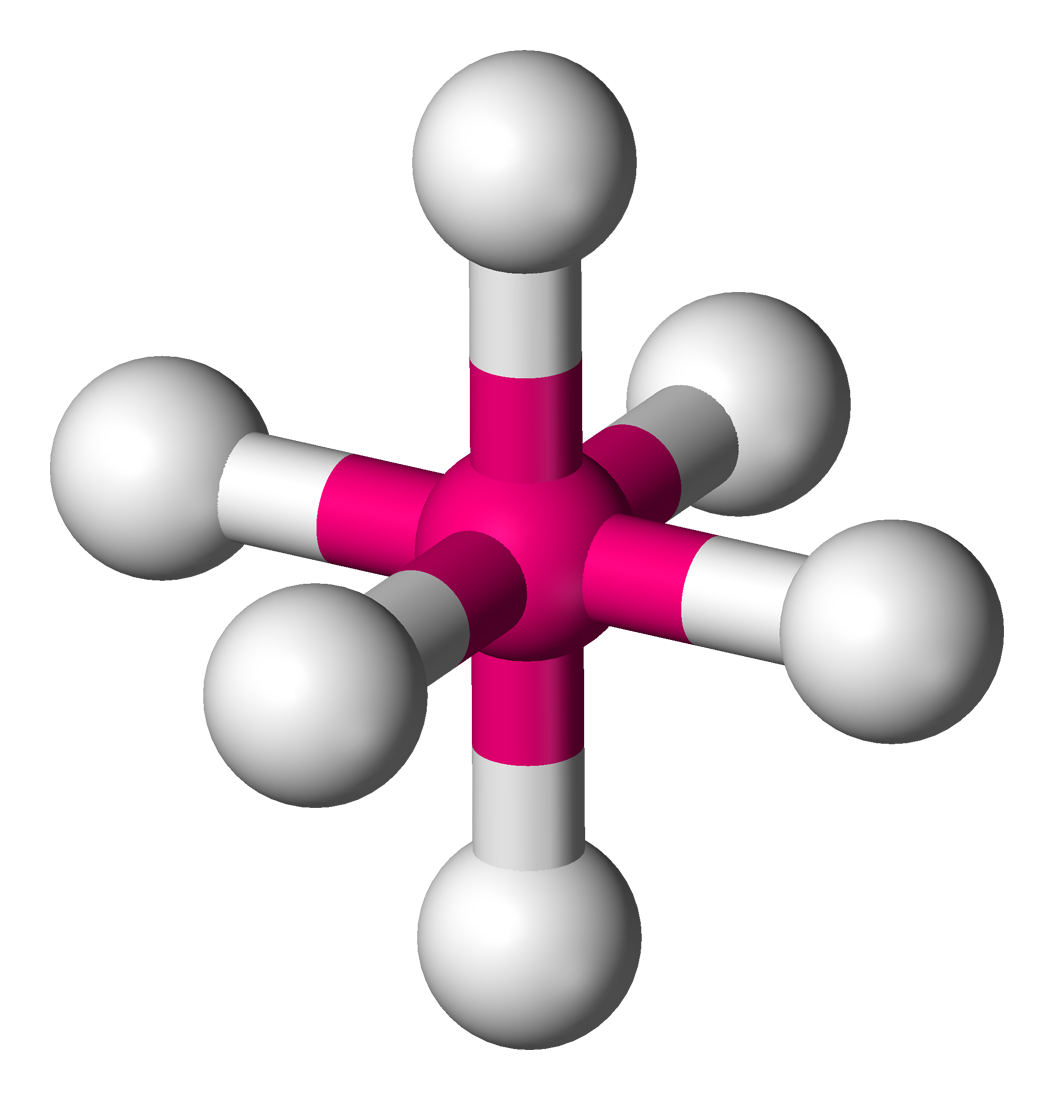
t shaped
5 electron domains, 2 lone pairs
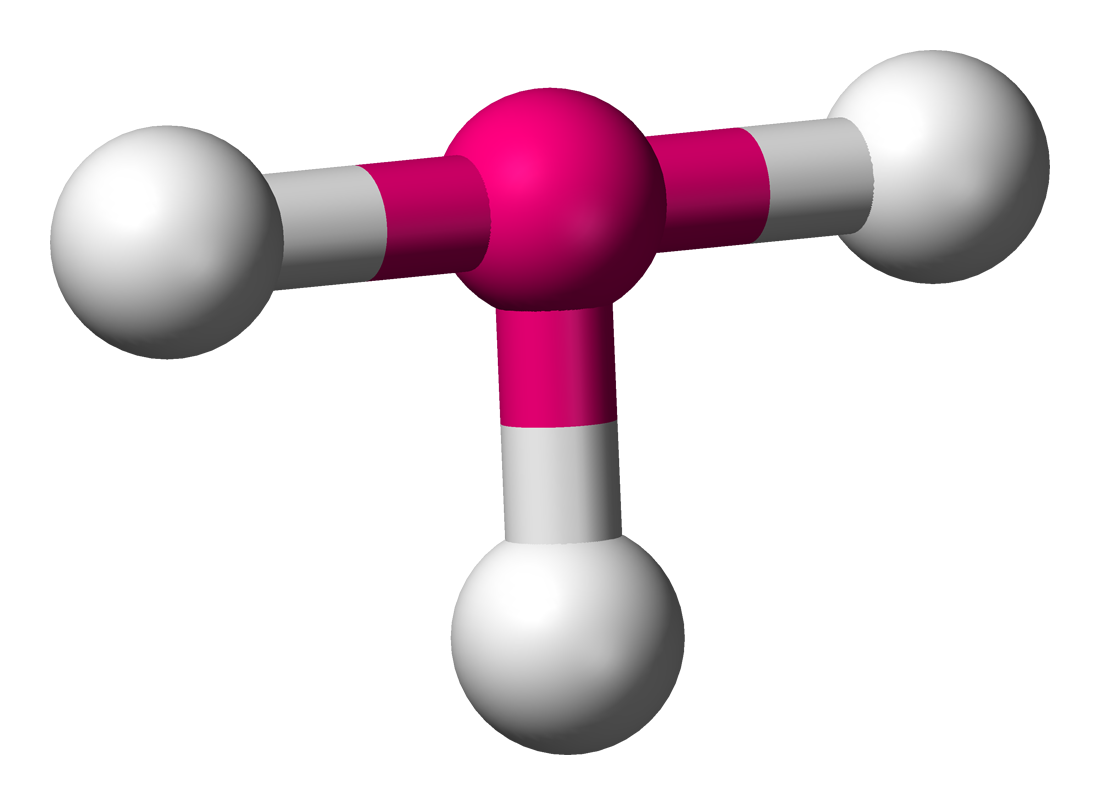
bent
3 electron domains, 1 lone pair
square planar
6 electron domains, 2 lone pairs
seesaw
5 electron domains, 1 lone pair
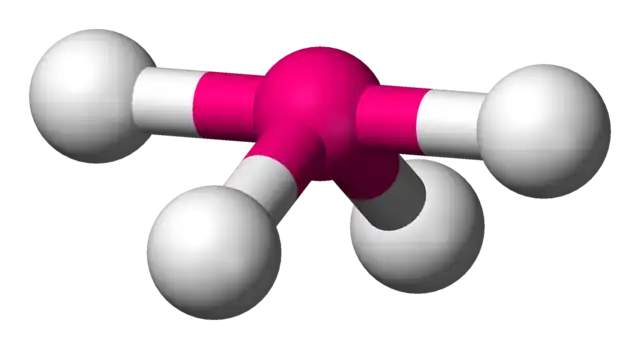
trigonal pyramidal
4 electron domains, 1 lone pair
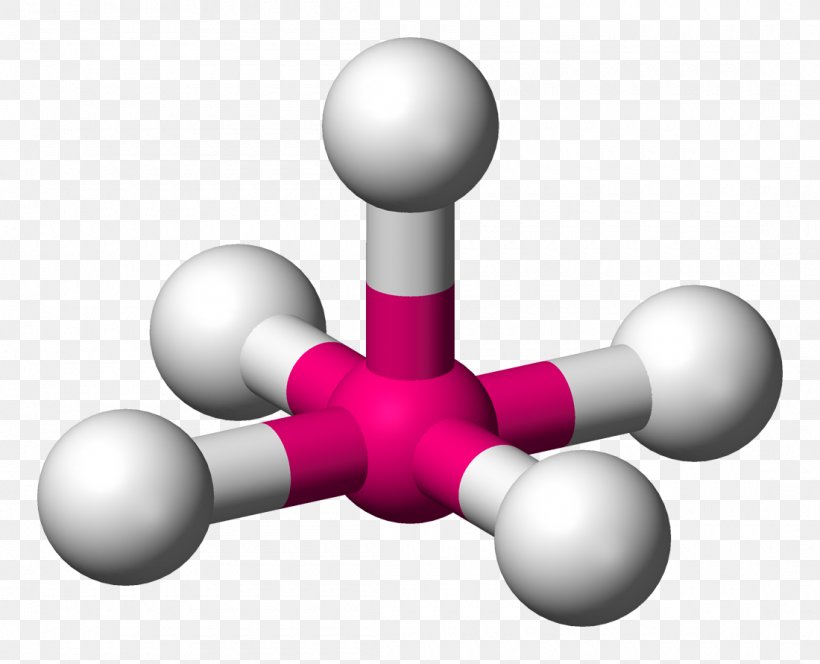
trigonal planar
3 electron domains, 0 lone pairs
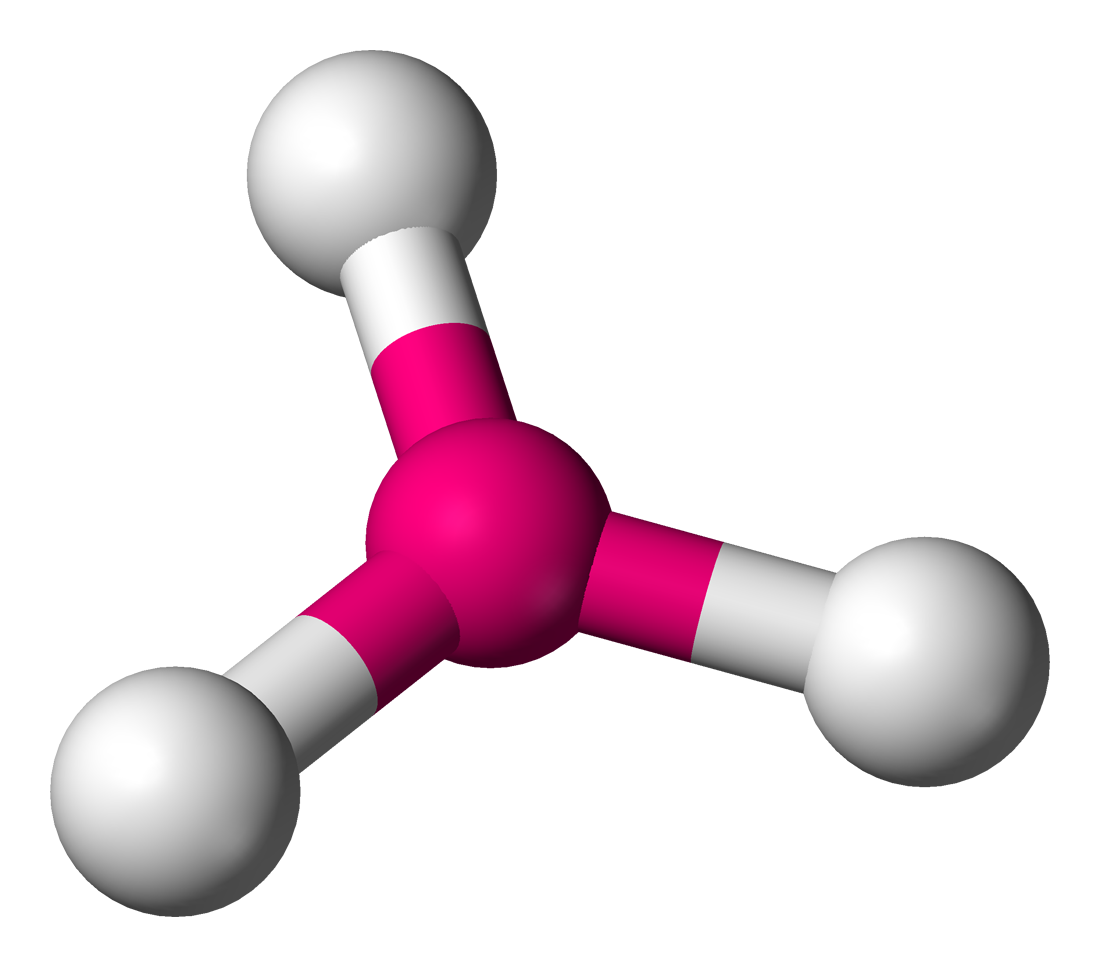
Square pyramidal
6 electron domains, 1 lone pair